El suelo vivo: un poco de lo que ocurre en este entorno. Un énfasis en los fitopatógenos
Contenido principal del artículo
Resumen
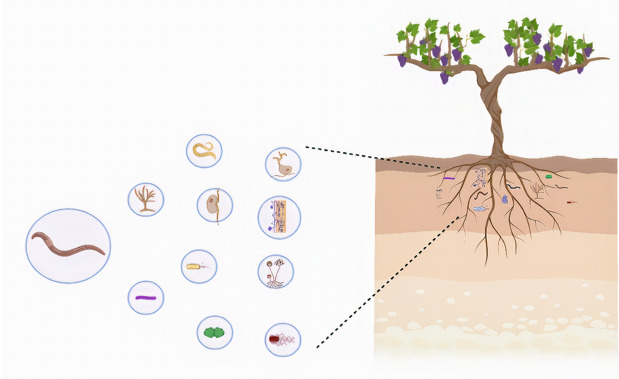
El suelo es un ecosistema con una reserva de carbono que sustenta la diversidad biológica. En esta revisión, presentamos cuán significativa es la simbiosis entre la raíz de la planta y los macro y microorganismos del suelo, así como los beneficios que genera para lograr un equilibrio ecológico y mantener bajas poblaciones de fitopatógenos en la producción de alimentos. Por ejemplo, las lombrices de tierra, colémbolos, cochinillas y ácaros oribátidos influyen, en gran medida, en el funcionamiento del sistema del suelo, ya que construyen y mantienen la estructura del suelo y participan activamente en el ciclo de nutrientes, a través de procesos de mineralización y humificación, además de consumir fitopatógenos. Por otro lado, microorganismos como los hongos micorrízicos, que se benefician al absorber los nutrientes de la planta, la ayudan a absorber los minerales del suelo y brindan protección a las raíces frente a los fitopatógenos. Los hongos micorrízicos inducen cambios en la planta y luego la planta responde produciendo exudados de las raíces que reducen o repelen a los patógenos. Otro ejemplo es el hongo Trichoderma, conocido como agente de biocontrol para la producción de metabolitos secundarios con actividad antimicrobiana contra fitopatógenos. Los agentes de control biológico y sus metabolitos secundarios son enfoques potenciales que se utilizan actualmente para reducir o reemplazar los agroquímicos. Finalmente, el manejo integrado de cultivos promueve la competencia y el equilibrio esenciales para mantener la salud del suelo y asegurar la producción de alimentos.
Detalles del artículo

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes de la Licencia CC Reconocimiento-NoComercial 4.0 Internacional (CC BY-NC 4.0):
Usted es libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia.
Bajo las condiciones siguientes:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
La revista no se responsabiliza con las opiniones y conceptos emitidos en los trabajos, son de exclusiva responsabilidad de los autores. El Editor, con la asistencia del Comité de Editorial, se reserva el derecho de sugerir o solicitar modificaciones aconsejables o necesarias. Son aceptados para publicar trabajos científico originales, resultados de investigaciones de interés que no hayan sido publicados ni enviados a otra revista para ese mismo fin.
La mención de marcas comerciales de equipos, instrumentos o materiales específicos obedece a propósitos de identificación, no existiendo ningún compromiso promocional con relación a los mismos, ni por los autores ni por el editor.
Citas
ONU. Objetivos del Desarrollo Sostenible de la Agenda 2030 ONU. 2021 [citado 20/07/2021]. Disponible en: https://www.un.org/sustainabledevelopment/
Avila-Quezada G, Silva-Rojas HV, Sánchez-Chávez E, Leyva-Mir G, Martínez-Bolaños L, Guerrero-Prieto V, et al. Seguridad alimentaria: la continua lucha contra las enfermedades de los cultivos. Tecnociencia Chihuahua. 2016;10(3):133-142.
Cintora-Martínez EA, Leyva-Mir SG, Ayala-Escobar V, Avila-Quezada G, Camacho-Tapia M, Tovar-Pedraza JM. Pomegranate fruit rot caused by Pilidiella granati in Mexico. Australasian Plant Disease Notes. 2017;12(1):4.
García-González T, Sáenz-Hidalgo HK, Silva-Rojas HV, Morales-Nieto C, Vancheva T, Koebnik R, et al. Enterobacter cloacae, an emerging plant-pathogenic bacterium affecting chili pepper seedlings. The Plant Pathology Journal. 2018;34(1):1-10.
Avila-Quezada GD, Esquivel JF, Silva-Rojas HV, Leyva-Mir G, García-Avila C, Noriega-Orozco L, et al. Emerging plant diseases under a changing climate scenario: Threats to our global food supply. Emirates Journal of Food and Agriculture. 2018;30(6):443-450.
Sánchez-Chávez E, Silva-Rojas HV, Leyva-Mir G, Villareal-Guerrero F, Jiménez-Castro J, Molina-Gayoso E, et al. An effective strategy to reduce the incidence of Phytophthora root and crown rot in bell pepper. Interciencia. 2017;42(4):229-235.
Galvez ZYA, Burbano VEM. Solubilización de fosfatos: una función microbiana importante en el desarrollo vegetal. NOVA Publicación en Ciencias Biomédicas. 2015;12(21):67-79.
Madrid-Delgado G, Orozco-Miranda M, Cruz-Osorio M, Hernández-Rodríguez A, Rodríguez-Heredia R, Roa-Huerta M, et al. Pathways of phosphorus absorption and early signaling between the mycorrhizal fungi and plants. Phyton International Jornal of Experimental Botany. 2021;90(5):1321-1338.
Le Bayon RC, Bullinger-Weber G, Schomburg A, Turberg P, Schlaepfer R, Guenat C. (2017). Earthworms as ecosystem engineers: A review. Earthworms: Types, Roles and Research. NOVA Science Publishers, New York, 129-178.
Sánchez-Rosales R, Hernández-Rodríguez A, Ojeda-Barrios D, Robles-Hernández L, González-Franco A, Parra-Quezada R. Comparison of three systems of decomposition of agricultural residues for the production of organic fertilizers. Chilean Journal of Agricultural Research. 2017;77(3):287-292.
Sulaiman ISC, Mohamad A. The use of vermiwash and vermicompost extract in plant disease and pest control. In: Natural Remedies for Pest, Disease and Weed Control. Academic Press; 2020. p. 187-201.
Andleeb S, Ejaz M, Awan UA, Ali S, Kiyani A, Shafique I, et al. In vitro screening of mucus and solvent extracts of Eisenia foetida against human bacterial and fungal pathogens. Pakistan Journal of Pharmaceutical Sciences. 2016;29(3):969-977.
Prakash M, Gunasekaran G. Antibacterial activity of the indigenous earthworms Lampito mauritii (Kinberg) and Perionyx excavatus (Perrier). The Journal of Alternative and Complementary Medicine. 2011;17:167-170.
Jouni F. Synergistic interaction earthworm-microbiota: a role in the tolerance and detoxification of pesticides?. Agricultural sciences. Université d’Avignon. 2018. English. ffNNT: 2018AVIG0699ff. [citado 20/07/2021]. Disponible en: https://tel.archives-ouvertes.fr/tel-02074579/document
Edwards CA, Fletcher KE. Interactions between earthworms and microorganisms in organic-matter breakdown. Agriculture, Ecosystems & Environment. 1988;24(1-3):235-247.
Nath G, Singh K. Combination of vermicomposts and biopesticides against nematode (Pratylenchus sp.) and their effect on growth and yield of tomato (Lycopersicon esculentum). IIOAB Journal. 2011;2:27-35.
Rostami M, Olia M, Arabi M. Evaluation of the effects of earthworm Eisenia fetida-based products on the pathogenicity of root-knot nematode (Meloidogyne javanica) infecting cucumber. International Journal of Recycling of Organic Waste in Agriculture. 2014;3(2):58.
Edwards CA, Arancon NQ, Emerson E, Pulliam R. Suppressing plant parasitic nematodes and arthropod pests with vermicompost teas. Biocycle. 2007;48(12):38-39.
Euteneuer P, Wagentristl H, Steinkellner S, Scheibreithner C, Zaller JG. Earthworms affect decomposition of soil-borne plant pathogen Sclerotinia sclerotiorum in a cover crop field experiment. Applied Soil Ecology. 2019;138:88-93.
Charles NJ, Martín Alonso NJ. Uso y manejo de hongos micorrízicos arbusculares (HMA) y humus de lombriz en tomate (Solanum lycopersicum L.), bajo sistema protegido. Cultivos Tropicales. 2015;36(1):55-64.
González-Escobedo R, Muñoz-Castellanos LN, Muñoz-Ramirez ZY, Guigón López C, Avila-Quezada GD. Microbial community analysis of rhizosphere of healthy and wilted pepper (Capsicum annuum L.) in an organic farming system. Microbial Ecology. 2021; Por asignar
Zhang H, Franken P. Comparison of systemic and local interactions between the arbuscular mycorrhizal fungus Funneliformis mosseae and the root pathogen Aphanomyces euteiches in Medicago truncatula. Mycorrhiza. 2014;24:419-430.
Song Y, Chen D, Lu K, Sun Z, Zeng R. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Frontiers in Plant Science. 2015;6:786.
Azcón R, Ambrosano E, Charest C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Science. 2003;165(5):1137-1145.
Chakravarty P, Unestam T. Differential influence of ectomycorrhizae on plant growth and disease resistance in Pinus sylvestris seedlings. Journal of Phytopathology. 1987;120(2):104-120.
Eke P, Adamou S, Fokom R, Nya VD, Fokou PVT, Wakam LN, et al. Arbuscular mycorrhizal fungi alter antifungal potential of lemongrass essential oil against Fusarium solani, causing root rot in common bean (Phaseolus vulgaris L.). Heliyon. 2020;6(12):e05737.
da Silva Campos MA. Bioprotection by arbuscular mycorrhizal fungi in plants infected with Meloidogyne nematodes: A sustainable alternative. Crop Protection. 2020;135:105203.
Sharma M, Saini I, Kaushik P, Al Dawsari MM, Al Balawi T, Alam P. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi Journal of Biological Sciences. 2021;28(7): 3685-3691.
Stummer BE, Zhang Q, Zhang X, Warren RA, Harvey PR. Quantification of Trichoderma afroharzianum, Trichoderma harzianum and Trichoderma gamsii inoculants in soil, the wheat rhizosphere and in planta suppression of the crown rot pathogen Fusarium pseudograminearum. Journal of Applied Microbiology. 2020; 129(4):971-990.
TariqJaveed M, Farooq T, Al-Hazmi AS, Hussain MD, Rehman AU. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. Journal of Invertebrate Pathology. 2021;107626.
Pozo-Serrano J, Cruz ERDL, Teresa-Cardoso M, Rodríguez-Pérez A, García-Pupo J, Pérez-Tejeda Y, et al. Efectividad antagónica In vitro de Trichoderma sp., frente a Stemphylium lycopersici. Cultivos Tropicales. 2019;40(3).
Köhl J, Kolnaar R, Ravensberg WJ. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Frontiers in Plant Science. 2019;10:845.
Vinale F, Sivasithamparam K, Ghisalberti EL, Woo SL, Nigro M, Marra R. Trichoderma secondary metabolites active on plants and fungal pathogens. The Open Mycology Journal. 2014;8(1):127-139.
Vinale F, Ghisalberti EL, Sivasithamparam K, Marral R, Ritieni A, Ferracane R, Woo S, Lorito M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Letters in Applied Microbiology. 2009;48(6):705-711.
Khan RAA, Najeeb S, Hussain S, Xie B, Li Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 2020;8(6), 817.
Sonkar P, Chandra R, Singh R, Kumar S. Study on management of Fusarium oxysporum through different mode of action of Trichoderma spp. International Journal of Current Trends Science and Technology. 2018;8:20192-20200.
Macheleidt J, Mattern DJ, Fischer J, Netzker T, Weber J, Schroeckh V, et al. Regulation and role of fungal secondary metabolites. Annual Review of Genetics. 2016;50:371-392.
Shyamli S, Prem D, Rs T, Atar S. Production and antifungal activity of secondary metabolites of Trichoderma virens. Pesticide Research Journal. 2005;17(2):26-29.
Shi M, Chen L, Wang XW, Zhang T, Zhao PB, Song XY, Sun CY, Chen XL, Zhou BC, Zhang YZ. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology. 2012;158:166-175.
Sha S, Liu L, Pan S, Wang WM. Isolation and purification of antifungal components from Trichoderma harzianum ferment broth by high-speed counter-current chromatography. Chinese Journal of Biological Control. 2013;29(1):83-88.
Marler TE, Krishnapillai MV. Vertical strata and stem carbon dioxide efflux in Cycas trees. Plants. 2020;9(2):230.
Chikov VI, Akhtyamova GA, Khamidullina LA. Ecological significance of the interaction of photosynthesis light and dark processes. American Journal of Plant Sciences. 2021;12(04):624.
Ferrero Holtz EW, Gonzalez MG, Giuffré L, Ciarlo E. Glomalins and their relationship with soil carbon. International Journal of Applied Science and Technology. 2016;6(2):69-73.
Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, et al. Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytologist. 2015;205(4):1537-1551.
Kittredge J. Soil Carbon Restoration: Can Biology do the Job?. NE Organic farming association, Massachusetts Chapter, 16. 2015. [citado 20/07/2021]. Disponible en: https://www.unifiedfieldcorporation.com/wp-content/uploads/2015/11/2015_White_Paper_web.pdf
Olivas‑Tarango AL, Tarango‑Rivero SH, Ávila‑Quezada GD. Pecan production improvement by zinc under drip irrigation in calcareous soils. Terra Latinoamericana, 2021;39:1-12.
Tarango-Rivero SH, Ávila-Quezada GD, Jacobo-Cuellar JL, Ramírez-Valdespino CA, Orrantia-Borunda E, Rodríguez-Heredia R, Olivas-Tarango AL. Chelated zinc and beneficial microorganisms: A sustainable fertilization option for pecan production. Revista Chapingo. Serie horticultura, 2022;28(3):145-159.