Residue decomposition of sugar cane harvest by an autochthonous fungal strain of Trichocladium pyriforme
Main Article Content
Abstract
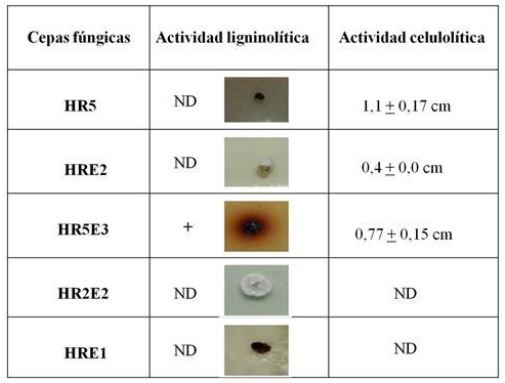
During the green harvesting system of sugarcane, a large amount of agricultural harvest residue is produced that can be left as mulch on the soil, removed from the field or incorporated into the profile, depending on the agroecological characteristics of each area. It is important to rapidly decompose the cover crop residue in areas where it is detrimental to sugarcane production. One of the alternatives to accelerate the natural decomposition of the residue is the use of lignocellulolytic fungi. The objective of this work was to isolate autochthonous fungal strains from sugarcane ARH, select and characterize culturally, morphologically and molecularly those with the greatest potential to accelerate the decomposition of the green harvest residue of the sugarcane field. Five autochthonous fungal strains were isolated from fragments of freshly harvested residue. Cellulolytic and ligninolytic activity was evaluated in vitro, using carboxymethylcellulose and guaiacol as substrates, respectively. Strain HR5E3 was the only strain able to decompose cellulose and lignin. This strain was culturally, morphologically and molecularly characterized as Trichocladium pyriforme and produced enzymes of the lignin peroxidase group, polyphenol oxidases and laccases. In solid substrate fermentation bioassays, this strain accelerated the decomposition of the residue by di-auxic growth with glucose. Trichocladium pyriforme HR5E3 could be used as a bioinoculant capable of degrading lignocellulose, and avoid the detrimental effects that the unaltered cover of the agricultural residue could have on the development of sugarcane.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Those authors who have publications with this journal accept the following terms of the License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0):
You are free to:
- Share — copy and redistribute the material in any medium or format
- Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
The journal is not responsible for the opinions and concepts expressed in the works, they are the sole responsibility of the authors. The Editor, with the assistance of the Editorial Committee, reserves the right to suggest or request advisable or necessary modifications. They are accepted to publish original scientific papers, research results of interest that have not been published or sent to another journal for the same purpose.
The mention of trademarks of equipment, instruments or specific materials is for identification purposes, and there is no promotional commitment in relation to them, neither by the authors nor by the publisher.
References
Fandos C, Scandaliaris P, Carreras- Baldrés J, Soria F, Giardina J. Área cosechable y producción de caña de azúcar y azúcar para la zafra 2020 en Tucumán. 2020;190:3-5.
Digonzelli PA, Romero ER, Alonso L, Ullivarri F, Quinteros R, Scandaliaris J, et al. Assessing a sustainable sugarcane production system in Tucumán, Argentina. Part 1: Dynamics of sugarcane harvest residue (trash) decomposition. Revista industrial y agrícola de Tucumán. 2011;88(1):1-12.
Carvalho JLN, Nogueirol RC, Menandro LMS, Bordonal R de O, Borges CD, Cantarella H, et al. Agronomic and environmental implications of sugarcane straw removal: a major review. GCB Bioenergy. 2017;9(7):1181-95. doi:https://doi.org/10.1111/gcbb.12410
Cherubin MR, Oliveira DM da S, Feigl BJ, Pimentel LG, Lisboa IP, Gmach MR, et al. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Scientia Agricola. 2018;75(3):255-72.
Marzi M, Shahbazi K, Kharazi N, Rezaei M. The Influence of Organic Amendment Source on Carbon and Nitrogen Mineralization in Different Soils. Journal of Soil Science and Plant Nutrition. 2020;20(1):177-91. doi:10.1007/s42729-019-00116-w
Maza M, Pajot HF, Amoroso MJ, Yasem MG. In-vitro degradation of Czapek and molasses amended post-harvest sugarcane residue by lignocellulolytic fungal strains. International Biodeterioration & Biodegradation. 2015;104:118-22. doi:10.1016/j.ibiod.2015.05.021
Maza M, Medina M, Plasencia AM, Amoroso MJ, Yasem MG. Fungal inoculation effect on post-harvest sugarcane residue decomposition under field conditions. Revista agronómica del noroeste argentino. 2018;38(2):65-73.
Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability. 2020;12(18):7282. doi:10.3390/su12187282
Ferreira JA, Mahboubi A, Lennartsson PR, Taherzadeh MJ. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresource Technology. 2016;215:334-45. doi:10.1016/j.biortech.2016.03.018
Kantharaj P, Boobalan B, Sooriamuthu S, Mani R. Lignocellulose degrading enzymes from fungi and their industrial applications. Int. J. Curr. Res. Rev. 2017;9(21):1-12.
Nguyen KA, Wikee S, Lumyong S. Brief review: lignocellulolytic enzymes from polypores for efficient utilization of biomass. Mycosphere. 2018;9(6):1073-88.
Ostengo S, Espinosa MA, Díaz JV, Chavanne ER, Costilla DD, Cuenya MI. Relevamiento de la distribución de variedades y de otras tecnologías aplicadas en el cultivo de caña de azúcar en la provincia de Tucumán: campaña 2016/2017. Gac. Agroindustrial EEAOC. 2018;81:14.
Sehgal J, Asha BM, Vardhan A, Siddalingeshwara KG. An Approach on Screening, Production and Characterization of Laccase from Fusarium. Journal of Current Pharma Research. 2020;10(2):3673-9.
Cruz-Hernández M. Avances de Investigación en Inocuidad de alimentos. In: Evaluación de hongos filamentosos para la producción de enzimas celulolíticas [Internet]. 2019. Available from: https://www.researchgate.net/publication/344359381_Evaluacion_de_hongos_filamentosos_para_la_produccion_de_enzimas_celuloliticas
Gaillard C, Strauss F. Ethanol precipitation of DNA with linear polyacrylamide as carrier. Nucleic Acids Research. 1990;18(2):378.
Ahmed PM, Pajot HF, de Figueroa LIC, Gusils CH. Sustainable bioremediation of sugarcane vinasse using autochthonous macrofungi. Journal of Environmental Chemical Engineering. 2018;6(4):5177-85. doi:10.1016/j.jece.2018.08.007
Pointing SB. Qualitative methods for the determination of lignocellulolytic enzyme production by tropical fungi. Fungal diversity. 1999;2:17-33.
Fonseca MI, Zapata PD, Villalba LL, Fariña JI. Characterization of the oxidative enzyme potential in wild white rot fungi from the subtropical forest of Misiones (Argentina). Acta biològica colombiana. 2015;20(1):47-56.
Goering HK, Soest PJV. Forage Fiber Analyses (apparatus, Reagents, Procedures, and Some Applications). Vol. 379. U.S. Agricultural Research Service; 1970. 24 p.
Dixon M. Trichocladium pyriformis sp. nov. Transactions of the British Mycological Society. 1968;51(1):160-4.
de Souza RSC, Okura VK, Armanhi JSL, Jorrín B, Lozano N, Da Silva MJ, et al. Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Scientific Reports. 2016;6(1):1-15.
Miura T, Niswati A, Swibawa IG, Haryani S, Gunito H, Shimano S, et al. Diversity of Fungi on Decomposing Leaf Litter in a Sugarcane Plantation and Their Response to Tillage Practice and Bagasse Mulching: Implications for Management Effects on Litter Decomposition. Microbial Ecology. 2015;70(3):646-58. doi:10.1007/s00248-015-0620-9
Spennati F, Ricotti A, Mori G, Siracusa G, Becarelli S, Gregorio SD, et al. The role of cosubstrate and mixing on fungal biofilm efficiency in the removal of tannins. Environmental Technology. 2020;41(26):3515-23. doi:10.1080/09593330.2019.1615128
Sen SK, Raut S, Bandyopadhyay P, Raut S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biology Reviews. 2016;30(3):112-33. doi:10.1016/j.fbr.2016.06.003
Beary TP, Boopathy R, Templet P. Accelerated decomposition of sugarcane crop residue using a fungal-bacterial consortium. International Biodeterioration & Biodegradation. 2002;50(1):41-6. doi:10.1016/S0964-8305(02)00056-2
Chu D, Barnes DJ. The lag-phase during diauxic growth is a trade-off between fast adaptation and high growth rate. Scientific Reports. 2016;6(1):1-15. doi:10.1038/srep25191
Durrant LR. Biodegradation of lignocellulosic materials by soil fungi isolated under anaerobic conditions. International Biodeterioration & Biodegradation. 1996;37(3):189-95. doi:10.1016/S0964-8305(96)00022-4
Pavarina EC, Durrant LR. Growth of lignocellulosic-fermenting fungi on different substrates under low oxygenation conditions. Applied Biochemistry and Biotechnology. 2002;98(1):663-77. doi:10.1385/ABAB:98-100:1-9:663