Benefits of co-inoculation of Arbuscular Mycorrhizal Fungi and rhizobia in bean cultivation
Main Article Content
Abstract
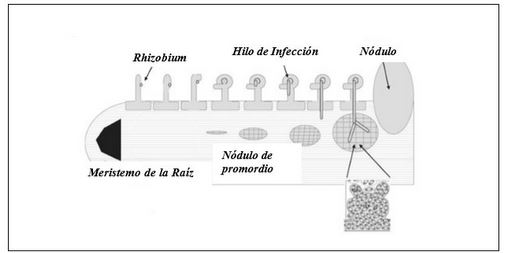
The common bean (Phaseolus vulgaris L.) is one of the leguminous plants of high human consumption. In Cuba, it constitutes one of the indispensable dishes in the Cuban menu, being the black bean the most common in Creole food. In spite of its importance and the fact that it is a traditional crop, it is necessary to increase the productivity of plants in a sustainable way, with little amount of resources and with the best quality standards, the national production still does not satisfy the consumption demand. Biofertilizers represent a sustainable, economically attractive and ecologically acceptable means to reduce external inputs and improve the quantity and quality of agricultural products, through the use of properly selected soil microorganisms, capable of making available to plants, through their biological activity. Among the microorganisms that have been most widely used for the development of biofertilizers are bacteria of the genus Rhizobium (rhizobia) and arbuscular mycorrhizal fungi (AMF). Rhizobia are nitrogen-fixing bacteria and plants, the formation of the specialized nodule, which ensures the reduction of atmospheric nitrogen, takes place in the root. Mycorrhizae are mutualistic symbiotic associations this interaction fungi benefit from the supply of carbon sources from the plant and the plant benefits from increased exploration of the soil, which enhances plant growth and development. However, the benefits of rhizobium-AMF co-inoculation in legumes of agricultural interest need further studies on these issues
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Those authors who have publications with this journal accept the following terms of the License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0):
You are free to:
- Share — copy and redistribute the material in any medium or format
- Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
The journal is not responsible for the opinions and concepts expressed in the works, they are the sole responsibility of the authors. The Editor, with the assistance of the Editorial Committee, reserves the right to suggest or request advisable or necessary modifications. They are accepted to publish original scientific papers, research results of interest that have not been published or sent to another journal for the same purpose.
The mention of trademarks of equipment, instruments or specific materials is for identification purposes, and there is no promotional commitment in relation to them, neither by the authors nor by the publisher.
References
Ulloa JA, Rosas Ulloa P, Ramírez Ramírez JC, Ulloa Rangel BE. El frijol (Phaseolus vulgaris): su importancia nutricional y como fuente de fitoquímicos. CONACYT [Internet]. 2011; Available from: http://dspace.uan.mx:8080/xmlui/handle/123456789/582
Chazan M. World prehistory and archaeology: pathways through time [Internet]. Routledge; 2021. Available from: https://www.routledge.com/World-Prehistory-and-Archaeology-Pathways-Through-Time/Chazan/p/book/9780367415686
Rivera R, Calderón A, Nápoles MC, Ruiz L, Mederos JD, Marrero Y, et al. La validación a escala productiva del biofertilizante EcoMic® y su aplicación conjunta con rizobios en el cultivo del frijol en el centro y occidente del país. Mayabeque, Cuba: INCA [Internet]. 2012; Available from: https://www.researchgate.net/publication/269993929_La_validacion_a_escala_productiva_del_biofertilizante_EcoMicR_y_su_aplicacion_conjunta_con_rizobios_en_el_cultivo_del_frijol_en_el_centro_y_occidente_del_pais
Martínez R, Dibut B. Biofertilizantes Bacterianos. La Habana, Cuba: Editorial Científico-Técnica; 279AD.
Gage DJ. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiology and molecular biology reviews [Internet]. 2004;68(2):280-300. doi:DOI: https://doi.org/10.1128/MMBR.68.2.280-300.2004
Werner AK, Sparkes IA, Romeis T, Witte C-P. Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiology [Internet]. 2008;146(2):418-30. Available from: https://pubmed.ncbi.nlm.nih.gov/18065556/
Sánchez C. Uso y manejo de los hongos micorrizógenos y abonos verdes en la producción de posturas de cafeto en algunos suelos del macizo Guamuhaya. Tesis en opción al Grado Científico de Doctor en Ciencias Agrícolas]. La Habana; 2001.
Peterson R, Mossicotte A, Milvelle L. Biofertilizantes, bioprotectores y biorestauradores Micorricicos para la producción agroecológica en las fincas de los Productores de café [Internet]. Feniagro. Nicaragua; 2010. Available from: http://www.renida.net.ni/renida/funica/REE14-F981b.pdf
Rivera Espinosa R, Fernández Suárez K. Bases científico-técnicas para el manejo de los sistemas agrícolas micorrizados eficientemente. In Instituto Nacional de Ciencias Agrícolas; 2003 [cited 21/01/2022]. Available from: http://repositorio.geotech.cu/jspui/handle/1234/3461
FAO. Food and Agriculture Statistics [Internet]. Food and Agriculture Organization of the United Nations. [cited 23/01/2022]. Available from: http://www.fao.org/food-agriculture-statistics/en/
Voysest O. Mejoramiento genético del frijol (Phaseolus vulgaris L.): Legado de variedades de América Latina 1930-1999 [Internet]. CIAT; 2000. 220 p. Available from: https://cgspace.cgiar.org/handle/10568/54161
Shi C, Chaudhary S, Yu K, Park SJ, Navabi A, McClean PE. Identification of candidate genes associated with CBB resistance in common bean HR45 (Phaseolus vulgaris L.) using cDNA-AFLP. Molecular biology reports [Internet]. 2011;38(1):75-81. Available from: https://link.springer.com/article/10.1007%2Fs11033-010-0079-1
Miklas P, Singh S. Genome Mapping and Molecular Breeding in Plants [Internet]. Springer. 2006 [cited 21/01/2022]. Available from: https://www.springer.com/series/7367
Vandemark GJ, Fourie D, Miklas PN. Genotyping with real-time PCR reveals recessive epistasis between independent QTL conferring resistance to common bacterial blight in dry bean. Theoretical and applied genetics [Internet]. 2008;117(4):513-22. Available from: https://pubmed.ncbi.nlm.nih.gov/18512042/
Alvarado A. Hemiptera-Pentatomidae en diferentes variedades de frijol común (Phaseolus vulgaris L.) en tres localidades de la provincia Villa Clara. [Universidad Central de las Villas (UCLV)]; 2009. 24 p.
ONEI. Anuario Estadístico de Cuba. Año 2018 [Internet]. Oficina Nacional de Estadística e Información, Sitio en Actualización. 2018 [cited 21/01/2022]. Available from: http://www.onei.gob.cu/node/13804
Martínez-González L, Maqueira-López L, Nápoles-García MC, Núñez-Vázquez M. Efecto de bioestimulantes en el rendimiento de dos cultivares de frijol (Phaseolus vulgaris L.) Biofertilizados. Cultivos Tropicales [Internet]. 2017;38(2):113-8. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362017000200017
Delgado R, Cabrera de Bisbal E, Ortega B, Velásquez L. Acumulación de materia seca, N, P y K en frijol cultivado bajo labranza mínima y convencional en un mollisol de Venezuela. Agronomía Tropical [Internet]. 2009;59(4):401-11. Available from: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0002-192X2009000400005
Valladares C. Taxonomía, Botánica y Fisiología de los cultivos de grano. Serie Lecturas Obligatorias [Internet]. Universidad Nacional Autónoma de Honduras. Centro Universitario Regional del Litoral Atlántico (CURLA); 2010. Available from: https://curlacavunah.files.wordpress.com/2010/04/unidad-ii-taxonomia-botanica-y-fisiologia-de-los-cultivos-de-grano-agosto-2010.pdf
Quintero E. Manejo agrotécnico del frijol en Cuba. Monografía. Facultad de Ciencias Agropecuarias, UCLV, Santa Clara. 2000;42.
Fallas R, Bertsch F, Echandi C, Henríquez C. Caracterización del desarrollo y absorción de nutrimentos del híbrido de maíz HC-57. Agronomía Costarricense [Internet]. 2011;35(2):33-47. Available from: https://www.scielo.sa.cr/scielo.php?pid=S0377-94242011000200003&script=sci_arttext
Haag WL, Adams MW, Wiersma JV. Differential Responses of Dry Bean Genotypes to N and P Fertilization of a Central American Soil 1. Agronomy journal [Internet]. 1978;70(4):565-8. Available from: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2134/agronj1978.00021962007000040014xa
Lata-Tenesaca L, Villaseñor-Ortiz D, Chabla-Carrillo J. Fraccionamiento de la absorción de nutrientes en cuatro etapas fenológicas del cultivo de Fréjol. Revista Universidad y Sociedad [Internet]. 2017;9(1):20-7. Available from: http://scielo.sld.cu/scielo.php?pid=S2218-36202017000100003&script=sci_arttext&tlng=en
Andrade MJB, Silva VMP, Carvalho JG, Vieira NMB, Junior JA. Pattern of nutrients absorption by common bean cv. BRS MG Talisma. Annual Report-Bean Improvement Cooperative [Internet]. 2005;48:162. Available from: http://arsftfbean.uprm.edu/bic/wp-content/uploads/2018/05/BIC_2005_volume_48.pdf#page=179
Hirzel C. Fertilización de cultivos en Chile. Colección Libros INIA-Instituto de Investigaciones Agropecuarias [Internet]. 2011; Available from: https://200.54.96.10/handle/123456789/3543
Arias J, Rengifo T, Jaramillo M. Buenas prácticas agrícolas en la producción de frijol voluble. Food and Agriculture Organization [Internet]. 2007;168. Available from: https://www.fao.org/3/a1359s/a1359s00.htm
Betancourt P, Pierre F. Extracción de macronutrientes por el cultivo de tomate (Solanum lycopersicum Mill. var. Alba) en casas de cultivo en Quíbor, estado Lara. Bioagro [Internet]. 2013;25(3):181-8. Available from: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1316-33612013000300005
Faure Alvarez B, Bentez Gonzÿlez R. Guía técnica para la producción de frijol común y maíz. Instituto de Investigaciones en Granos, Artemisa (Cuba); 2014 p. 7-21.
MINAGRI. Informe del diagnóstico de la cadena del frijol en la región central Cuba. 2015.
Paredes FL, Millo EP. Influencia de la fertilización nitrogenada en la contaminación por nitratos de las aguas subterráneas. Levante Agrícola: Revista internacional de cítricos [Internet]. 1992;(317):4-14. Available from: https://dialnet.unirioja.es/servlet/articulo?codigo=4639176
Martínez-Viera R, Dibut B, Yoania R. Efecto de la integración de aplicaciones agrícolas de biofertilizantes y fertilizantes minerales sobre las relaciones suelo-planta. Cultivos Tropicales [Internet]. 2010;31(3):27-31. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362010000300009
Barea J, Azcón-Aguilar C. Microbial co-operation in the rhizosphere | Journal of Experimental Botany | Oxford Academic [Internet]. 2005 [cited 21/01/2022]. Available from: https://academic.oup.com/jxb/article/56/417/1761/484466
Alvarez BD. Biofertilizantes como insumos en agricultura sostenible [Internet]. INIFAT, Cub: Editorial Universitaria (Cuba); 2009. Available from: https://books.google.es/books?hl=es&lr=&id=--vzDwAAQBAJ&oi=fnd&pg=PP2&dq=Biofertilizantes+como+insumos+en+Agricultura+Sostenible&ots=DzqnNhsgz6&sig=-hhXdGgFpLv9m5p5Z-v8qrAZhxs#v=onepage&q=Biofertilizantes%20como%20insumos%20en%20Agricultura%20Sostenible&f=false
Martínez-González L, Reyes-Guerrero Y, Falcón-Rodríguez A, Nápoles-García MC, Núñez-Vázquez M de la C. Efecto de productos bioactivos en plantas de frijol (Phaseolus vulgaris L.) biofertilizadas. Cultivos Tropicales [Internet]. 2016;37(3):165-71. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362016000300018&script=sci_arttext&tlng=en
Urquiaga S, Zapata F. Manejo eficiente de la fertilización nitrogenada de cultivos anuales en América Latina y el Caribe, Segundo Urquiaga, Felipe Zapata, eds. 2000;110. Available from: https://biblioteca.epn.edu.ec/cgi-bin/koha/opac-detail.pl?biblionumber=23790
Deaker R, Roughley RJ, Kennedy IR. Legume seed inoculation technology. A review. Soil biology and biochemistry [Internet]. 2004;36(8):1275-88. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0038071704001269
Mayea S, Novo R, Valiño A. Introducción a la microbiología del suelo. Editorial Pueblo y Educación, La Habana. 1982;
Bauer T. Microorganismos fijadores de nitrógeno: Familia rhizobiaceae. fgranea@ microbiología. com. 2001;
Jiménez-Zacarías JJ, Peña-Cabriales JJ. Fijación biológica de N2 (FBN) en leguminosas de América Latina. La fijación biológica de nitrógeno en América Latina: El aporte de las técnicas isotópicas. 2000;
García M, Treto E, Álvarez M. Los abonos verdes: una alternativa para la economía del nitrógeno en el cultivo de la papa. II. Efecto de la interacción abono verde-dosis de nitrógeno. Cultivos tropicales [Internet]. 2000;21(1):13-9. Available from: https://www.redalyc.org/pdf/1932/193232232002.pdf
Giller KE. Nitrogen fixation in tropical cropping systems [Internet]. Cabi; 2001. Available from: https://www.cabi.org/ISC/ebook/20013118217
Long SR. Genes and signals in the Rhizobium-legume symbiosis. Plant physiology [Internet]. 2001;125(1):69-72. Available from: https://academic.oup.com/plphys/article/125/1/69/6098962?login=true
Ouma EW, Asango AM, Maingi J, Njeru EM. Elucidating the potential of native rhizobial isolates to improve biological nitrogen fixation and growth of common bean and soybean in smallholder farming systems of Kenya. International Journal of Agronomy [Internet]. 2016;2016:1-7. Available from: https://www.hindawi.com/journals/ija/2016/4569241/
Nkot LN, Fankem H, Adamou S, Ngakou A, Nwaga D, Etoa F-X. Abundance of legume nodulating bacteria in soils of diverse land use systems in Cameroon. Universal Journal of Plant Science [Internet]. 2015;3(5):97-108. Available from: https://www.researchgate.net/profile/Ngakou-Albert-2/publication/290996824_Abundance_of_Legume_Nodulating_Bacteria_in_Soils_of_Diverse_Land_Use_Systems_in_Cameroon/links/569d476408aed27a702f9dea/Abundance-of-Legume-Nodulating-Bacteria-in-Soils-of-Diverse-Land-Use-Systems-in-Cameroon.pdf
Colás-Sánchez A, Díaz-Pérez B, Rodríguez-Urrutia A, Gatorno-Muñóz S, Rodríguez López O. Efecto de la biofertilización en la morfo fisiología y rendimiento del frijol común (Phaseolus vulgaris L.). Centro Agrícola [Internet]. 2018;45(4):34-42. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-57852018000400034
Mercante FM, Otsubo AA, Brito OR. New native rhizobia strains for inoculation of common bean in the Brazilian savanna. Revista Brasileira de Ciência do Solo [Internet]. 2017;41. Available from: https://www.scielo.br/j/rbcs/a/qkFXhNRKPpmgtY5krCVz55C/?lang=en
C N, Gretel G, Costales D, Freixas-Coutin J, Guevara E, Meira S, et al. Signals in Soybean’s Inoculants. In 2011. doi:10.5772/14882
Martínez Viera R, López M, Dibut B, Parra C, Rodríguez J. La fijación biológica del nitrógeno atmosférico en el medio tropical. Ed. MPPAT, Caracas. 2007;190.
Pueppke SG, Broughton WJ. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Molecular Plant-Microbe Interactions [Internet]. 1999;12(4):293-318. Available from: https://apsjournals.apsnet.org/doi/abs/10.1094/MPMI.1999.12.4.293
Wais RJ, Keating DH, Long SR. Structure-function analysis of nod factor-induced root hair calcium spiking in Rhizobium-legume symbiosis. Plant physiology [Internet]. 2002;129(1):211-24. Available from: https://academic.oup.com/plphys/article/129/1/211/6110204?login=true
Kinkema M, Scott PT, Gresshoff PM. Legume nodulation: successful symbiosis through short-and long-distance signalling. Functional Plant Biology [Internet]. 2006;33(8):707-21. Available from: https://www.publish.csiro.au/fp/fp06056
Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial symbiosis. Annual review of genetics [Internet]. 2011;45:119-44. Available from: https://www.annualreviews.org/doi/abs/10.1146/annurev-genet-110410-132549
Hayashi S, Gresshoff PM, Ferguson BJ. Mechanistic action of gibberellins in legume nodulation. Journal of integrative plant biology [Internet]. 2014;56(10):971-8. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jipb.12201
Russelle MP, Schepers JS, Raun WR. Biological dinitrogen fixation in agriculture. Agronomy [Internet]. 2008;49:281-359. Available from: https://books.google.es/books?hl=es&lr=&id=an-LKuRRmwoC&oi=fnd&pg=PA281&dq=Biological+Dinitrogen+Fixation+in+Agriculture.+Nitrogen+in+Agricultural+Systems&ots=oBH1-TWIw7&sig=GVO7Yrm3_sE9snFKJzrze-RroDg#v=onepage&q=Biological%20Dinitrogen%20Fixation%20in%20Agriculture.%20Nitrogen%20in%20Agricultural%20Systems&f=false
Ferguson BJ, Indrasumunar A, Hayashi S, Lin M-H, Lin Y-H, Reid DE, et al. Molecular analysis of legume nodule development and autoregulation. Journal of integrative plant biology [Internet]. 2010;52(1):61-76. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1744-7909.2010.00899.x
Martínez R, López M, Dibut B, Parra C, Rodríguez J. La fijación del nitrógeno atmosférico en el medio tropical, Ed. MPPAT, Caracas. 2007;190.
Freixas JA, Reynaldo IM, Nápoles MC. Influencia de la sequía sobre el metabolismo del nitrógeno fijado durante la simbiosis Bradyrhizobium-soya. Cultivos Tropicales [Internet]. 2010;31(2):00-00. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362010000200009&script=sci_arttext&tlng=pt
González JES, Cuéllar AE, Espinosa RR. Manejo, integración y beneficios del biofertilizante micorrízico EcoMic® en la producción agrícola. Agricultura Tropical [Internet]. 2021;6(3). Available from: https://www.researchgate.net/publication/340223155_Manejo_integracion_y_beneficios_del_biofertilizante_micorrizico_EcoMicR_en_la_produccion_agricola
Alvarado A, Chavarría M, Guerrero R, Boniche J, Navarro JR. Características edáficas y presencia de micorrizas en plantaciones de teca (Tectona grandis Lf) en Costa Rica. Agronomía Costarricense [Internet]. 2004;28(1):89-100. Available from: https://www.redalyc.org/pdf/436/43628109.pdf
Entry JA, Rygiewicz PT, Watrud LS, Donnelly PK. Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Advances in Environmental Research [Internet]. 2002;7(1):123-38. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1093019101001095
Linderman RG. Vesicular‐arbuscular mycorrhizae and soil microbial interactions. Mycorrhizae in sustainable agriculture [Internet]. 1992;54:45-70. Available from: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2134/asaspecpub54.c3
Benizri E, Baudoin E, Guckert A. Root colonization by inoculated plant growth-promoting rhizobacteria. Biocontrol science and technology [Internet]. 2001;11(5):557-74. Available from: https://www.tandfonline.com/doi/abs/10.1080/09583150120076120
Gamper H, Hartwig UA, Leuchtmann A. Mycorrhizas improve nitrogen nutrition of Trifolium repens after 8 years of selection under elevated atmospheric CO2 partial pressure. New Phytologist [Internet]. 2005;167(2):531-42. Available from: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/j.1469-8137.2005.01440.x
Janos DP. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza [Internet]. 2007;17(2):75-91. Available from: https://link.springer.com/article/10.1007%2Fs00572-006-0094-1
Smith SE, Read DJ. Mycorrhizal symbiosis [Internet]. Academic press; 2010. Available from: https://books.google.es/books?hl=es&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP1&dq=Mycorrhizal+symbiosis&ots=zrvSoTSykK&sig=abuTQELXzzJjWe_ADZ1KTc1kpAs#v=onepage&q=Mycorrhizal%20symbiosis&f=false
Allen MF, Swenson W, Querejeta JI, Egerton-Warburton LM, Treseder KK. Ecology of mycorrhizae: a conceptual framework for complex interactions among plants and fungi. Annual Review of Phytopathology [Internet]. 2003;41(1):271-303. Available from: https://www.annualreviews.org/doi/abs/10.1146/annurev.phyto.41.052002.095518
Rivera R, Ruiz L, Fernández F, Sánchez C, Riera M, Hernández A, et al. La simbiosis micorrízica efectiva y el sistema suelo-planta-fertilizante. In: Congreso Sociedad Cubana de la Ciencia del Suelo (6: 2006 mar 8-10: La Habana). Memorias.[CD-ROM] Ciudad de la Habana: Centro de Convenciones Capitolio [Internet]. 2006. Available from: https://www.researchgate.net/profile/Ramon-Espinosa-3/publication/301624642_La_SimbiosisS_Micorrizica_Efectiva_y_el_Sistema_Suelo-Planta-_Fertilizante/links/571e1b8808aefa6488999769/LA-SIMBIOSIS-MICORRIZICA-EFECTIVA-Y-EL-SISTEMA-SUELO-PLANTA-FERTILIZANTE.pdf
Ibarra-Puón JC, Aguirre-Medina JF, Coss L-D, Cadena-Iñiguez J, Zavala-Mata GA. Coffea canephora (Pierre) ex Froehner inoculado con micorriza y bacteria fijadora de nitrógeno en vivero. Revista Chapingo. Serie horticultura [Internet]. 2014;20(2):201-13. Available from: http://www.scielo.org.mx/scielo.php?pid=S1027-152X2014000200006&script=sci_abstract&tlng=pt
Rivera R, Fernández F, Fernández K, Ruiz L, Sánchez C, Riera M. Advances in the management of effective arbuscular mycorrhizal symbiosis in tropical ecosystesm. Mycorrhizae in crop production [Internet]. 2007;151-96. Available from: https://www.researchgate.net/profile/Ramon-Espinosa-3/publication/269993713_Advances_in_the_management_of_effective_arbuscular_mycorrhizal_symbiosis_in_tropical_ecosystesm/links/550c4e3f0cf2ac2905a3c2fb/Advances-in-the-management-of-effective-arbuscular-mycorrhizal-symbiosis-in-tropical-ecosystesm.pdf
Espinosa-Cuéllar A, Rivera-Espinosa R, Ruiz-Martínez L, Espinosa-Cuéllar E, Lago-Gato Y. Manejo de precedentes inoculados con HMA para micorrizar eficientemente el boniato Ipomoea batatas (L.) Lam en sucesión. Cultivos Tropicales [Internet]. 2019;40(2). Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362019000200003&script=sci_arttext&tlng=pt
Marrero-Cruz YJ, Rivera Espinosa RA. Efecto de frecuencias de inoculación micorrízica y el laboreo sobre una secuencia de cultivos en un suelo Pardo Mullido Carbonatado [Internet]. [Instituto Nacional de Ciencias Agrícolas, Cuba]; 2010. 78 p. Available from: http://repositorio.geotech.cu/jspui/bitstream/1234/2843/2/Efecto%20de%20frecuencias%20de%20inoculaci%C3%B3n%20micorr%C3%ADzica%20y%20laboreo%20sobre%20una%20secuencia%20de%20cultivos%20en%20suelo%20pardo.pdf
Covacevich F, Marino MA, Echeverría HE. The phosphorus source determines the arbuscular mycorrhizal potential and the native mycorrhizal colonization of tall fescue and wheatgrass. European Journal of Soil Biology [Internet]. 2006;42(3):127-38. Available from: https://www.sciencedirect.com/science/article/abs/pii/S116455630500076272.
Leigh J, Hodge A, Fitter AH. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist [Internet]. 2009;181(1):199-207. Available from: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/j.1469-8137.2008.02630.x
Villegas J, Fortin JA. Phosphorus solubilization and pH changes as a result of the interactions between soil bacteria and arbuscular mycorrhizal fungi on a medium containing NO3-as nitrogen source. Canadian Journal of Botany [Internet]. 2002;80(5):571-6. Available from: https://cdnsciencepub.com/doi/abs/10.1139/b02-038
Gryndler M. Interactions of arbuscular mycorrhizal fungi with other soil organisms. In: Arbuscular mycorrhizas: Physiology and function [Internet]. Springer; 2000. p. 239-62. Available from: https://link.springer.com/chapter/10.1007/978-94-017-0776-3_11
López-Pedrosa A, González-Guerrero M, Valderas A, Azcón-Aguilar C, Ferrol N. GintAMT1 encodes a functional high-affinity ammonium transporter that is expressed in the extraradical mycelium of Glomus intraradices. Fungal Genetics and Biology [Internet]. 2006;43(2):102-10. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1087184505001660
Cardoso IM, Kuyper TW. Mycorrhizas and tropical soil fertility. Agriculture, ecosystems & environment [Internet]. 2006;116(1-2):72-84. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0167880906001149
Helgason T, Fitter AH. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum glomeromycota). Journal of experimental botany [Internet]. 2009;60(9):2465-80. Available from: https://academic.oup.com/jxb/article/60/9/2465/513679?login=true
Atul-Nayyar A, Hamel C, Hanson K, Germida J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza [Internet]. 2009;19(4):239-46. Available from: https://link.springer.com/article/10.1007%2Fs00572-008-0215-0
Requena N, Breuninger M, Franken P, Ocón A. Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant physiology [Internet]. 2003;132(3):1540-9. Available from: https://academic.oup.com/plphys/article/132/3/1540/6111703?login=true
Yao Q, Zhu H-H, Hu Y-L, Li L-Q. Differential influence of native and introduced arbuscular mycorrhizal fungi on growth of dominant and subordinate plants. Plant Ecology [Internet]. 2008;196(2):261-8. Available from: https://link.springer.com/article/10.1007/s11258-007-9350-5
Ezawa T, Cavagnaro TR, Smith SE, Smith FA, Ohtomo R. Rapid accumulation of polyphosphate in extraradical hyphae of an arbuscular mycorrhizal fungus as revealed by histochemistry and a polyphosphate kinase/luciferase system. New Phytologist [Internet]. 2004;161(2):387-92. Available from: https://nph.onlinelibrary.wiley.com/doi/full/10.1046/j.1469-8137.2003.00966.x
Koide RT, Kabir Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytologist [Internet]. 2000;148(3):511-7. Available from: https://nph.onlinelibrary.wiley.com/doi/abs/10.1046/j.1469-8137.2000.00776.x
Wang S, Lin X, Yin R, Hou Y. Effect of inoculation with arbuscular mycorrhizal fungi on the degradation of DEHP in soil. Journal of Environmental Sciences [Internet]. 2004;16(3):458-61. Available from: https://content.iospress.com/articles/journal-of-environmental-sciences/jes16-3-23
González PJ, Ramírez JF, Rivera R, Hernández A, Plana R, Crespo G, et al. Management of arbuscular mycorrhizal inoculation for the establishment, maintenance and recovery of grasslands. Revista Cubana de Ciencia Agrícola [Internet]. 2015;49(4):535-40. Available from: https://www.redalyc.org/pdf/1930/193045908016.pdf
Martínez LAR, Cuéllar AE, Hernández MC, González JS, Espinosa RR. Efecto de dosis de nitrógeno, fósforo y potasio combinadas con micorrizas sobre el cultivo de la yuca en un suelo pardo mullido carbonatado. Agricultura Tropical [Internet]. 2017;2(2). Available from: https://www.researchgate.net/publication/326129315_Efecto_de_dosis_de_Nitrogeno_Fosforo_y_Potasio_combinadas_con_micorrizas_sobre_el_cultivo_de_la_yuca_en_un_suelo_Pardo_mullido_carbonatado
Novo R. Curso internacional de Microbiología del Suelo, los Biofertilizantes y la Biofertilización. Asociación de Ingenieros Agrónomos Colombianos residentes en el Ecuador (ASOINCO). Quito, agosto. 2002;19:58.
Ventimiglia LA, Torrens Baudrix L. Inoculación y coinoculación en soja: sumando esfuerzos para mejorar el rendimiento [Internet]. AER 9 de Julio, EEA Pergamino, INTA; 2019. Available from: https://repositorio.inta.gob.ar/handle/20.500.12123/6593
Bonfante P, Anca I-A. Plants, mycorrhizal fungi and bacteria: a network of interactions. Annual review of microbiology [Internet]. 2009;63:363-83. Available from: https://www.annualreviews.org/doi/abs/10.1146/annurev.micro.091208.073504
Sodré Filho J, Cardoso AN, Carmona R, Carvalho AM de. Fitomassa e cobertura do solo de culturas de sucessão ao milho na Região do Cerrado. Pesquisa Agropecuária Brasileira [Internet]. 2004;39:327-34. Available from: https://www.scielo.br/j/pab/a/YSmFRnWsfH6zFrJqTMHrk8w/abstract/?lang=pt
Dodd JC, Rosendahl S, Giovannetti M, Broome A, Lanfranco L, Walker C. Inter‐and intraspecific variation within the morphologically‐similar arbuscular mycorrhizal fungi Glomus mosseae and Glomus coronatum. New Phytologist [Internet]. 1996;133(1):113-32. Available from: https://nph.onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-8137.1996.tb04347.x
Alfonso ET, Galán AL. Evaluación agrobiológica de la coinoculación micorrizas-rizobacterias en tomate. Agronomía Costarricense [Internet]. 2006;30(1):65-73. Available from: https://revistas.ucr.ac.cr/index.php/agrocost/article/view/6832
Martín GM, Reyes R, Ramírez JF. Coinoculación de Canavalia ensiformis (L.) DC con Rhizobium y Hongos micorrÍzicos arbusculares en dos tipos de suelos de Cuba. Cultivos Tropicales [Internet]. 2015;36(2):22-9. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362015000200004
Terry Alfonso E, Ruiz Padrón J, Tejeda Peraza T, Díaz de Armas MM. Respuesta del cultivo de la habichuela (Phaseolus vulgaris L. var. Verlili.) a la aplicación de diferentes bioproductos. Cultivos Tropicales [Internet]. 2013;34(3):05-10. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362013000300001
Corbera J, Nápoles MC. Evaluación de la inoculación conjunta Bradyrhizobium japonicum-hongos MA y la aplicación de un bioestimulador del crecimiento vegetal en soya, cultivada en época de primavera. Cultivos Tropicales [Internet]. 2011;32(4):13-9. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362011000400002&script=sci_arttext&tlng=pt
Flores GC, Ramírez JF, González PJ, Hernández I. Coinoculación de cepas de rizobios y del hongo micorrízico arbuscular en Stylosanthes guianensis vc. CIAT-184. Revista Cubana de Ciencia Agrícola [Internet]. 2014;48(3):297-300. Available from: https://www.redalyc.org/pdf/1930/193032133016.pdf