Hongos micorrizógenos arbusculares y Azotobacter chroococcum en la producción de posturas de cocotero
Contenido principal del artículo
Resumen
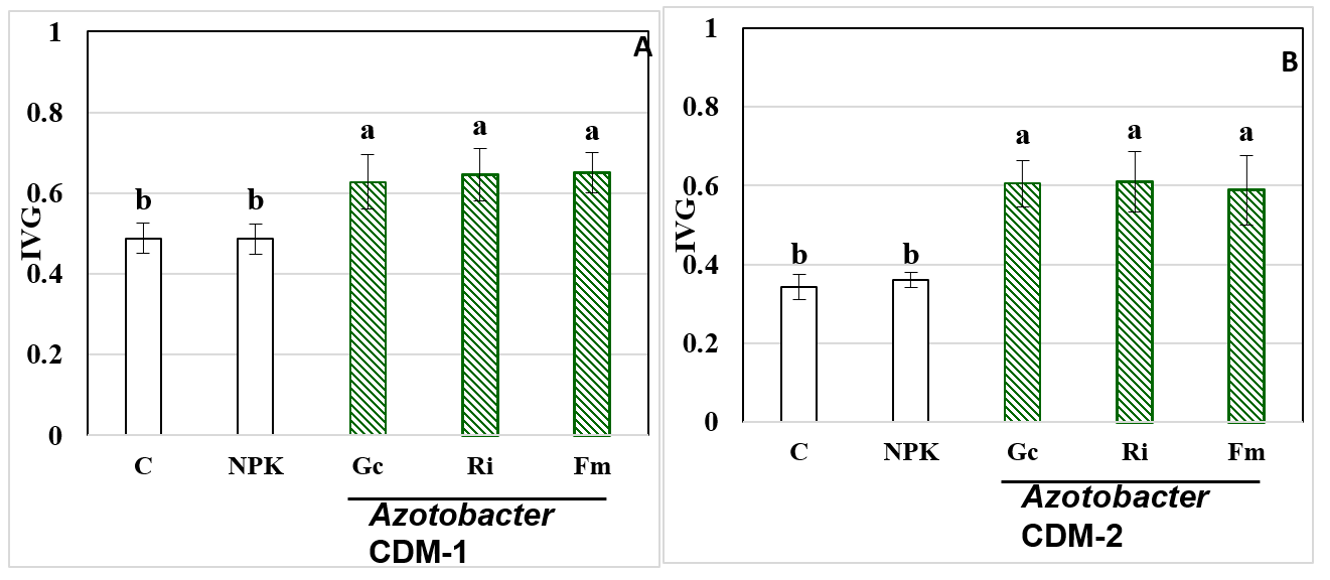
El cocotero es un cultivo extendido, con importancia en la alimentación, la cosmética y la industria; sin embargo, su producción se ve afectada, entre otros factores, por el envejecimiento de las plantaciones, lo cual disminuye los niveles de productividad, por lo cual se hace imprescindible la obtención de posturas de calidad para la repoblación de las áreas de producción. Con el objetivo de estudiar el efecto combinado de los hongos micorrizógenos arbusculares (HMA) y cepas de Azotobacter en la producción de posturas de cocotero de alta calidad, se desarrolló un experimento en dos viveros de Baracoa, Guantánamo, Cuba, del cual se realizaron dos repeticiones en el tiempo. Se empleó el ecotipo domesticado de cocotero “Indio Verde-1” en dos suelos: Arenosol háplico (ARh) y Gleysol Flúvico háplico (GFLh), con la inoculación de tres cepas de HMA: Glomus cubense, Rhizophagus irregularis y Funneliformis mosseae y dos cepas de Azotobacter: A. chroococcum (CDM-1 aislada del suelo ARh y CDM-2 aislada del suelo GFLh. Se observó una respuesta diferencial de las especies de HMA en ambos suelos. La inoculación con R. intraradices favoreció el incremento de las variables germinación de las semillas, altura, diámetro del vástago y número de hojas, en el suelo ARh al combinarse su inoculación con la cepa A. chroococcum CDM-1, mientras que G. cubense fue el mejor tratamiento en el suelo GFLh, combinado con A. chroococcum CDM-2. A pesar de que se cuantificaron elevados niveles de las cepas autóctonas de HMA, en ambos suelos, las cepas inoculadas demostraron ser más eficientes que estas para la obtención de posturas de cocotero de calidad.
Detalles del artículo

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes de la Licencia CC Reconocimiento-NoComercial 4.0 Internacional (CC BY-NC 4.0):
Usted es libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia.
Bajo las condiciones siguientes:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
La revista no se responsabiliza con las opiniones y conceptos emitidos en los trabajos, son de exclusiva responsabilidad de los autores. El Editor, con la asistencia del Comité de Editorial, se reserva el derecho de sugerir o solicitar modificaciones aconsejables o necesarias. Son aceptados para publicar trabajos científico originales, resultados de investigaciones de interés que no hayan sido publicados ni enviados a otra revista para ese mismo fin.
La mención de marcas comerciales de equipos, instrumentos o materiales específicos obedece a propósitos de identificación, no existiendo ningún compromiso promocional con relación a los mismos, ni por los autores ni por el editor.
Citas
Qadi WSM, Mediani A, Benchoula K, Wong EH, Misnan NM, Sani NA. Characterization of Physicochemical, Biological, and Chemical Changes Associated with Coconut Milk Fermentation and Correlation Revealed by 1H NMR-Based Metabolomics. Foods. 2023, 12(10): 1971. doi:10.3390/foods12101971.
Wirkijowska A, Sobota A, Zarzycki P, Nawrocka A, Blicharz-Kania A y Andrejko D. Chemical, technological, and sensory evaluation of the suitability of coconut by-products in white rolls. J Sci Food Agric. 2022; 102(8):3370-3378. doi: 10.1002/jsfa.11684.
Saraiva A, Carrascosa C, Ramos F, Raheem D, MariaLopes M y Raposo A. coconut sugar: chemical analysis and nutritional profile; health impacts; safety and quality control; food industry applications. Int J Environ Res Public Health. 2023, 19;20(4):3671. doi: 10.3390/ijerph20043671.
Divya PM, Roopa BS, Manusha C y Balannara P. A concise review on oil extraction methods, nutritional and therapeutic role of coconut products. Review J Food Sci Technol. 2023, 60(2):441-452. doi:10.1007/s13197-022-05352-0.
Kaushik V, Chogale R y Mhaskar S. Single hair fiber assessment techniques to discriminate between mineral oil and coconut oil effect on hair physical properties. Journal of Cosmet Dermatol. 2021; 20(4), 1306–1317. doi:10.1111/jocd.13724.
Singh S, Lohani A, Mishra AK y Verma A. Formulation and evaluation of carrot seed oil-based cosmetic emulsions. Journal of Cosmetic and Laser Therapy. 2019; 21(2), 99-107. doi:10.1080/14764172.2018.1469769.
Sumit AF, Sharmin T y Ahmed T. Evaluation of the in vitro antimicrobial activity as well as preservative capacity of several popular cosmetic products available in the neighbouring shops in Bangladesh. Mymensingh Medical Journal. 2021; 30(2), 478-484. PMID: 33830132.
Limones BV, Fernández BMA. El cocotero: “El árbol de la vida”. Herbario CICY. 2016; 8:107–110. Available from: http://www.cicy.mx/sitios/desde_herbario/.
Gopal M, Gupta A, Arunachalam V, Maheswarappa HP, Thomas GV, Jacob PM. Autochthonous nutrient recycling driven by soil microbiota could be sustaining high coconut productivity in Lakshadweep Islands sans external fertilizer application. World J Microbiol Biotechnol. 2022; 2;38(11):213. doi: 10.1007/s11274-022-03373-7.
Tuckeldoe RB, Maluleke MK y Adriaanse P. The effect of coconut coir substrate on the yield and nutritional quality of sweet peppers (Capsicum annuum) varieties. Sci Rep. 2023; 15;13(1):2742. doi: 10.1038/s41598-023-29914-0.
Masijn Q, Libberecht S, Meyfroot A, Goemaere O, Hanskens J y Fraeye I. Structure and physical stability of plant-based food gel systems: Impact of protein (mung bean, pea, potato, soybean) and fat (coconut, sunflower). Heliyon. 2023; 6; 9(9):e18894. doi: 10.1016/j.heliyon.2023.e18894.
Deen A, Visvanathan R, Wickramarachchi D, Marikkar N, Nammi S, Jayawardanae BC y Liyanagea R. Chemical composition and health benefits of coconut oil: an overview. Journal of the Science of Food and Agriculture. 2021; 101(6), 2182-2193. doi:10.1002/jsfa.10870.
Fang C, Paul CR, Day CH, Chang R, Kuo C, Ho T, Hsieh DJ, Viswanadha VP, Kuo W y Huang C. Poria cocos (Fuling) targets TGFβ /Smad7 associated collagen accumulation and enhances Nrf2‐antioxidant mechanism to exert anti‐skin aging effects in human dermal fibroblasts. Environmental Toxicolog. 2020; 36(5), 729–736. doi:10.1002/tox.23075.
Neelakantan N, Seah JYH y van Dam RM. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Clinical Trials Circulation. 2020; 141(10), 803-814. doi:10.1161/circulationaha.119.043052.
Schwingshackl L, Schlesinger S. Coconut Oil and Cardiovascular Disease Risk. Curr Atheroscler Rep. 2023; 25(5):231-236. doi: 10.1007/s11883-023-01098-y.
Spiazzi BF, Duarte AC, Zingano CP, Teixeira PP, Amazarray CR, Merello EN, Wayerbacher LF, Farenzena LP, Correia PE, Bertoluci MC, Gerchman F, Colpani V. Coconut oil: an overview of cardiometabolic effects and the public health burden of misinformation. Arch Endocrinol Metab. 2023; 19;67(6):e000641. doi: 10.20945/2359-3997000000641.
Teng M, Zhao YJ, Khoo AL, Yeo TC, Yong QW y Lim BP. Impact of coconut oil consumption on cardiovascular health: a systematic review and meta-analysis. Nutrition Reviews. 2020; 78(3), 249-259. doi:10.1093/nutrit/nuz074.
Cuerda-Ballester M, Proaño B, Alarcón-Jimenez J, Bernardo N, Villaron-Casales C, Lajara Romance JM, Rubia Ortí JE. Improvements in gait and balance in patients with multiple sclerosis after treatment with coconut oil and epigallocatechin gallate. A pilot study. Food Funct. 2023, 23;14(2):1062-1071. doi:10.1039/d2fo02207a.
Rethinam P. Chapter 2: International Scenario of Coconut Sector. In: Nampoothiri KUK, Krishnakumar V, Thampan PK, Achuthan Nair M, editors. The Coconut Palm (Cocos nucifera L.) - Research and Development Perspectives. 2018. pp 21-56. Singapore: Springer. doi:10.1007/978-981-13-2754_2.
Samosir YMS y Adkins SW. Improving acclimatization through the photoautotrophic culture of coconut (Cocos nucifera) seedlings: an in vitro system for the efficient exchange of germplasm. In Vitro Cell Dev Plant. 2014; 50:493–501. doi: 10.1007/s11627-014-9599-z.
Thomas GV, Krishnakumar V, Dhanapal R y Reddy DVS. Chapter 7 Agro-management Practices for Sustainable Coconut Production. . In: Nampoothiri KUK, Krishnakumar V, Thampan PK, Achuthan Nair M, editors. The Coconut Palm (Cocos nucifera L.) - Research and Development Perspectives. 2018. (pp 227-322). Singapore: Springer. doi:10.1007/978-981-13-2754_7.
Vafa ZN, Sohrabi Y, Sayyed RZ, Luh Suriani N, y Datta R. Effects of the Combinations of Rhizobacteria, Mycorrhizae, and Seaweed, and Supplementary Irrigation on Growth and Yield in Wheat Cultivars. Plants. 2021; 10(4), 811. doi:10.3390/plants10040811.
Vishwakarma K, Kumar N, Shandilya C, Mohapatra S, Bhayana S y Varma A. Revisiting Plant–Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020; 11:560406. doi: 10.3389/fmicb.2020.560406.
Siim KS, Teele J, Martti V, Martin Z, Maarja Ö. Effects of land use on arbuscular mycorrhizal fungal communities in Estonia. Mycorrhiza. 2018; 28 (3): 259–268. DOI 10.1007/s00572-018-0822-3.
Müller LM y Harrison MJ. Phytohormones, miRNAs, and peptide signals integrate plant phosphorus status with arbuscular. mycorrhizal symbiosis. Current Opinion in Plant Biology. 2019; 50, 132–139. doi:10.1016/j.pbi.2019.05.004.
Sarmiento-López LG, López-Meyer M, Sepúlveda-Jiménez G, Cárdenas L y Rodríguez-Monroy M. Photosynthetic performance and stevioside concentration are improved by the arbuscular mycorrhizal symbiosis in Stevia rebaudiana under different phosphate concentrations. Peer J. 2020; 18p. doi: 10.7717/peerj.10173.
Rajeshkumar PP, Thomas GV, Gupta A y Gopal M. Diversity, richness and degree of colonization of arbuscular mycorrhizal fungi in coconut cultivated along with intercrops in high productive zone of Kerala, India. Symbiosis. 2015; 65,125–14. doi:10.1007/s13199-015-0326-2.
Martin Del Campo JS, Rigsbee J, Bueno Batista M, Mus F, Rubio LM, Einsle O, Peters JW, Dixon R, Dean DR y Dos Santos PC. Overview of physiological, biochemical, and regulatory aspects of nitrogen fixation in Azotobacter vinelandii. Crit Rev Biochem Mol Biol. 2022;57(5-6):492-538. doi: 10.1080/10409238.2023.2181309.
Sumbul A, Ansari RA, Rizvi R y Mahmood I. Azotobacter: A potential bio-fertilizer for soil and plant health management. Saudi Journal of Biological Sciences. 2020; 27, 3634-3640. doi: 10.1016/j.sjbs.2020.08.004.
Carmona A, Reyes JJ, Chiquito RG, Rincon G, Cerdan CR y Hernández LG. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A Review. Agronomy. 2021; 9, 121. doi:10.3390/agronomy9030121.
Kalapchieva S, Tringovska I, Bozhinova R, Kosev V, Hristeva T. Population response of rhizosphere microbiota of garden pea genotypes to inoculation with arbuscular mycorrhizal fungi. Int J Mol Sci. 2023; 6;24(2):1119. doi: 10.3390/ijms24021119.
Lamlom SF, Irshad A, Mosa WFA. The biological and biochemical composition of wheat (Triticum aestivum) as affected by the bio and organic fertilizers. BMC Plant Biol. 2023; 23;23(1):111. doi: 10.1186/s12870-023-04120-2.
Sagar A, Rathore P, Ramteke PW, Ramakrishna W, Reddy MS y Pecoraro L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms. 2021; 9, 1491. doi:10.3390/ microorganisms9071491.
Shi J, Wang X, Wang E. Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annu Rev Plant Biol. 2023; 22;74:569-607. doi: 10.1146/annurev-arplant-061722-090342.
Wahab A, Muhammad M, Munir A, Abdi G, Zaman W, Ayaz A, Khizar C y Reddy SPP. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants. (Basel) 2023; 29;12(17):3102. doi: 10.3390/plants12173102.
Alvarado K, Blanco A, Martín GM, Ríos Y, Capdesuñer R, Matos K, de la Noval BM. Influencia de un sistema de abonado orgánico y Azotobacter chroococcum sobre posturas de cocotero. Cultivos Tropicales. 2019; 40(1): a06-e06. Available from: https://ediciones.inca.edu.cu/index.php/ediciones/article/view/1495
MINAG. Instructivo técnico para el cultivo del COCO. 1ra Edición, edit. Biblioteca ACTAF, Ciudad de la Habana. 2011, 15p. Available from: http://actaf.co.cu/index.php.
Paneque PVM, Calaña NJM, Calderón VM, Borges BY, Hernández GTC y Caruncho CM. Manual de técnicas analíticas para análisis de suelo, foliar, abonos orgánicos y fertilizantes químicos.1st ed. La Habana, Cuba: Ediciones INCA. 2010. Available from: http://mst.ama.cu/578/.
Herrera PRA, Furrazola E, Ferrer RL, Fernández VR y Torres Y. Functional strategies of root hair sand arbuscular mycorrhizae in an evergreen tropical forest, Sierra del Rosario, Cuba. Revista CENIC Ciencias Biológicas. 2004; 35(2),113-123. Available from: http://www.redalyc.org/articulo.oa?id=181226079010.
Alonso, M., Cueto, J.R., Santos, Y., Romero, W., LLauger, R. y Rohde, W. (2007). Variabilidad morfológica y molecular de una población de cocoteros verdes en la región de Baracoa. Cultivos Tropicales. 28(3), 69-75. Available from: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://ediciones.inca.edu.cu/index.php/ediciones/article/download/293/pdf/813&ved=2ahUKEwjrla_53ZCJAxU_TDABHXhBA6MQFnoECBgQAQ&usg=AOvVaw3NkCnmnalNP5YOZQV6CY5i
Rodríguez Y, Dalpé Y, Séguin S, Fernández K, Fernández F, Rivera RA. Glomus cubenses p. nov., an arbuscular mycorrhizal fungus from Cuba. Mycotaxon. 2011; 118(1): 337-347. doi 10.5248/118.337.
Shüßler A, Walker C. The Glomeromycota: a species list with new families and new genera. Gloucester: The Royal Botanic Garden Edinburgh, The Royal Botanic Garden Kew, Botanische Staatssammlung Munich, and Oregon State University. 2011, 58p. Available from: https://books.google.com.cu/books/about/The_Glomeromycota.html?id=qjILywAACAAJ&redir_esc=y
Sabio C. Evaluación de Mycoral® en tres variedades de cocotero resistentes al Amarillamiento Letal del cocotero para mejorar el crecimiento en condiciones de vivero [en línea] [Tesis para optar por el título de Ingeniero Agrónomo], Escuela Agrícola panamericana. Zamorano, Honduras. 2002; 37 p. [Consultado: 25 de mayo de 2012], Available from: http://www.bdigital.zamorano.edu/bitstream/11036/2209/1/cpa.2002- T101.
Fernández F, Gómez R, Vanegas LF, Noval BM, Martínez MA. Producto inoculante micorrizógeno; Cuba; Oficina Nacional de Propiedad Industrial Patente No. 22641, 2000. Available from: https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=ED1D72BCCE74B2DBD801D4D94327D0B1.wapp2nA?docId=CU4401498yrecNum=98yoffice=yqueryString=yprevFilter=%26fq%3DOF%3ACU%26fq%3DICF_M%3A%22A01N%22ysortOption=Pub+Date+DescymaxRec=135.
Paneque VM, Calaña JM. La fertilización de los cultivos. Aspectos teórico-prácticos para su recomendación. Departamento de Biofertilizantes y Nutrición de las Plantas. INCA. La Habana. 2001; 29 p. Available from: http://www.inca.edu.cu.
Giovanetti M, Mosse B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologyst. 1980; 84 pp. 489-500. doi:10.1111/j.1469-8137.1980.tb04556.x.
Furrazola E, Covacevich F, Torres AY, Rodríguez RRM, Ley RJF, Izquierdo K, Fernández VR, Louro BRL. Functionality of arbuscular mycorrhizal fungi in three plant communities in the Managed Floristic Reserve San Ubaldo-Sabanalamar, Cuba. International Journal Tropical Biology. 2015; 63 (2): 341-356. ISSN: 0034-7744.
Furrazola GE, Rodríguez RRM, Torres AY, González GS, Ortega FR, Ley RJF. Hongos micorrizógenos arbusculares (Glomeromycotina) en ecosistemas naturales y agrícolas en la Reserva de la Biosfera Ciénaga de Zapata, Cuba. Acta Botánica Cubana. 2018; 217 (1): 85-93. Available from: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.revistasgeotech.com/index.php/abc/article/view/225&ved=2ahUKEwiAqoy_3pCJAxWnkoQIHXNzAA8QFnoECBIQAQ&usg=AOvVaw1LAldsCh34oBDB0JBRBBnc
Kumar BS, Bandyopadhyay P, Kumar P, Kumar MD, Prasad R, Kumari A, Chandra UK, Kumar YP, Varma A. Interaction of Piriformospora indica with Azotobacter chroococcum. Scientific Reports. 2015; 5(13911):1-13. doi:10.1038/srep13911.
Khalid M, Hassani D, Bilal M, Asad F y Huang D. Influence of bio fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Botanical Studies. 2017; 58, 35. doi:10.1186/s40529-017-0189-3.
Lara-Pérez LA, Oros-Ortega I, Córdova-Lara I, Estrada-Medina H, O'Connor-Sánchez A, Góngora-Castillo E, Sáenz-Carbonell L. Seasonal shifts of arbuscular mycorrhizal fungi in Cocos nucifera roots in Yucatán, México. Mycorrhiza. 2020; 30(2-3):269-283. doi: 10.1007/s00572-020-00944-0.
Pandey S y Gupta S. Diversity analysis of ACC deaminase producing bacteria associated with rhizosphere of coconut tree (Cocos nucifera L.) grown in Lakshadweep islands of India and their ability to promote plant growth under saline conditions. J Biotechnol. 2020; 20;324:183-197. doi: 10.1016/j.jbiotec.2020.10.024.
Ayamba BE, Abaidoo RC, Opoku A, Ewusi-Mensah N. Mechanisms for nutrient interactions from organic amendments and mineral fertilizer inputs under cropping systems: a review. Peer J. 2023; 4;11:e15135. doi: 10.7717/peerj.15135.
Khanal U, Wilson C, Rahman S, Lee BL y Hoang VN. Smallholder farmers'adaptation to climate change and its potential contribution to UN's sustainable development goals of zero hunger and no poverty. Journal of Cleaner Production. 2021; 281:124999. doi 10.1016/j.jclepro.2020.124999.
Sadvakasova AK, Kossalbayev BD, Token AI, Bauenova MO, Wang J, Zayadan BK, Balouch H, Alwasel S, Leong YK, Chang JS y Allakhverdiev SI. Influence of Mo and Fe on Photosynthetic and Nitrogenase Activities of Nitrogen-Fixing Cyanobacteria under Nitrogen Starvation. Cells. 2022; 5;11(5):904. doi: 10.3390/cells11050904.
Ortíz J, Sanhueza C, Romero-Munar A, Hidalgo-Castellanos J, Castro C, Bascuñán-Godoy L, Coba de la Peña T, López-Gómez M, Florez-Sarasa I y Del-Saz NF. In Vivo Metabolic Regulation of Alternative Oxidase under Nutrient Deficiency-Interaction with Arbuscular Mycorrhizal Fungi and Rhizobium Bacteria. Int J Mol Sci. 2020; 12;21(12):4201. doi: 10.3390/ijms21124201.
Santana LR, da Silva LN, Tavares GG, Batista PF, Cabral JSR y Souchie EL. Arbuscular mycorrhizal fungi associated with maize plants during hydric deficit. Sci Rep. 2023; 27;13(1):1519. doi: 10.1038/s41598-023-28744-4.
Sethi D, Subudhi S, Rajput VD, Kusumavathi K, Sahoo TR, Dash S, Mangaraj S, Nayak DK, Pattanayak SK, Minkina T, Glinushkin AP y Kalinitchenko VP. Exploring the role of mycorrhizal and rhizobium inoculation with organic and inorganic fertilizers on the nutrient uptake and growth of Acacia mangium saplings in acidic soil. Forests. 2021; 12(12):1657. doi:10.3390/f12121657.
Hodge A, Storer K. Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant and Soil. 2015; 386 (1-2): 1-19. doi: 10.1007/s11104-014-2162-1.
Bahrani A, Pourreza J, Hagh JM. Response of winter wheat to co-inoculation with Azotobacter and Arbuscular Mycorrhizal Fungi (AMF) under Different Sources of Nitrogen Fertilizer. American-Eurasian Journal Agricultural and Environment Sciences. 2010; 8(1):95-103. ISSN: 1818-6769.
Rico GMA. Capacidad promotora de crecimiento vegetal por bacterias del género Azotobacter y Actinomicetos aislados de cultivos de Solanum tuberosum Linnaeus, 1753 (Papa) cultivados en zonas altoandinas del Perú. [en línea] [Tesis en opción título profesional de Biólogo con Mención en Biología Celular y Genética] Facultad de Ciencias Biológica. E. A. P. de Ciencias Biológicas. Universidad Nacional Mayor De San Marcos. Lima, Perú. 2009; 152 p. [Consultado: 20 de enero de 2018], Available from: .
Rivera R, González PJ, Hernández JA, Martín G, Ruíz L, Fernández K, Simó J, García M, Pérez A, Riera M, Bustamante C, Joao JP, Ruiz M. La importancia del ambiente edáfico y del pH sobre la efectividad y la recomendación de cepas eficientes de HMA para la inoculación de los cultivos. En: VIII Congreso de la Sociedad Cubana de la Ciencia del Suelo, del 2 al 5 de Junio. La Habana, Cuba. 2015. Available from: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.researchgate.net/publication/279193397_La_importancia_del_ambiente_edafico_y_del_pH_sobre_la_efectividad_y_la_recomendacion_de_cepas_eficientes_de_HMA_para_la_inoculacion_de_los_cultivos&ved=2ahUKEwiU5eT-3pCJAxV3RjABHd23Ii8QFnoECBIQAQ&usg=AOvVaw1TvPMKoIGSMdAb_xGqEv4h
Zhang W, Li XG, Sun K, Tang MJ, Xu FJ, Zhang M y Dai CC. Mycelial network-mediated rhizobial dispersal enhances legume nodulation. ISME J. 2020; 4(4):1015-1029. doi: 10.1038/s41396-020-0587-5.
Huang D, Ma M, Wang Q, Zhang M, Jing G, Li C y Ma F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol Biochem. 2020; 149:245-255. doi: 10.1016/j.plaphy.2020.02.020.
Ilangamudali IMPS, Senarathne SHS. Effectiveness of Arbuscular Mycorrhizal Fungi based biofertilizer on early growth of coconut seedlings. COCOS. 2016; 22: 1-12. doi: 10.4038/cocos.v22i1.5807.
Pokluda R, Ragasová L, Jurica M, Kalisz A, Komorowska M, Niemiec M y Sekara A. Effects of growth promoting microorganisms on tomato seedlings growing in different media conditions. PLoS ONE. 2021; 16(11): e0259380. doi:10.1371/journal.pone.0259380.
Mesbah R, Ardakani MR, Moghaddam A y Rafiei F. Yield and morpho-physiological traits of tobacco (Nicotiana tabacum L.) as affected by azotobacter, mycorrhizal symbiosis and biochar application. Plant Science Today. 2021; 8(4), 986–994. doi:10.14719/pst.1378.