Chitosan and its derivatives, natural polymers with potential for control of Pyricularia oryzae (Cav.)
Main Article Content
Abstract
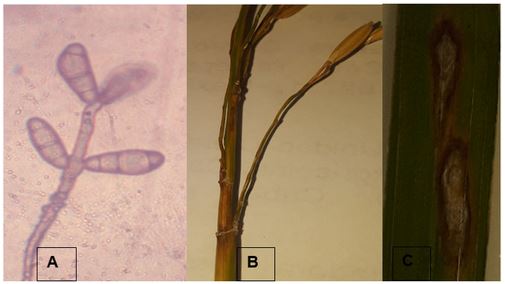
Chitosan and its derivatives are natural compounds that have potential in agriculture for the control of one of the rice diseases, pyriculariosis (Pyricularia oryzae), of great importance worldwide. In general, this disease is controlled with synthetic fungicides belonging to the benzimidazole group; however, their use has generated adverse results to the environment, together with the low sensitivity of the fungus to them. This article provides a review of published research on chitosan, its physicochemical characteristics, general information on the fungus P. oryzae, the fungicidal action of chitosan and its derivatives in in vitro and in situ research on this fungus, and in general, the possible mechanisms of action of this compound.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Those authors who have publications with this journal accept the following terms of the License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0):
You are free to:
- Share — copy and redistribute the material in any medium or format
- Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
The journal is not responsible for the opinions and concepts expressed in the works, they are the sole responsibility of the authors. The Editor, with the assistance of the Editorial Committee, reserves the right to suggest or request advisable or necessary modifications. They are accepted to publish original scientific papers, research results of interest that have not been published or sent to another journal for the same purpose.
The mention of trademarks of equipment, instruments or specific materials is for identification purposes, and there is no promotional commitment in relation to them, neither by the authors nor by the publisher.
References
Prashanth KV, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential-an overview. Trends in Food Science & Technology. 2007;18(3):117-31. doi:10.1016/j.tifs.2006.10.022
Ramos-García M de L, Bautista-Baños S, Barrera-Necha LL, Bosquez-Molina E, Alia-Tejacal I, Estrada-Carrillo M. Compuestos antimicrobianos adicionados en recubrimientos comestibles para uso en productos hortofrutícolas. Revista mexicana de fitopatología. 2010;28(1):44-57.
Kumar S, Mukherjee A, Dutta J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends in Food Science & Technology. 2020;97:196-209.
Xing K, Zhu X, Peng X. Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agronomy for Sustainable Development. 2015;35(2):569-88.
Rodríguez AT, Ramírez MA, Nápoles MC, Márquez R, Cárdenas RM. Antifungal activity of chitosan and one of its hydrolysates on Pyricularia grisea, Sacc. Fungus. Cultivos Tropicales. 2003;24(2):85-8.
Rodríguez AT, Ramírez MA, Cárdenas RM, Hernández AN, Velázquez MG, Bautista S. Induction of defense response of Oryza sativa L. against Pyricularia grisea (Cooke) Sacc. by treating seeds with chitosan and hydrolyzed chitosan. Pesticide Biochemistry and Physiology. 2007;89(3):206-15.
Cárdenas RM, Ramírez MA, Rodríguez AT, González LM. Efecto de los derivados de quitina y su combinación con sulfato de cobre en el comportamiento del crecimiento micelial y esporulación de un aislamiento monospórico del hongo Pyricularia grisea Sacc. Cultivos Tropicales. 2004;25(4):89-93.
Manikandan A, Sathiyabama M. Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. International journal of biological macromolecules. 2016;84:58-61.
Muzzarelli RAA. Enzymatic synthesis of chitin and chitosan. Occurrence of chitin. Chitin. 1977;5-17.
Zhang N, Luo J, Rossman AY, Aoki T, Chuma I, Crous PW, et al. Generic names in Magnaporthales. IMA fungus. 2016;7(1):155-9.
Lugo L, Jayaro Y, González Á, Borges O. Identification of sources of partial resistance to Pyricularia grisea in rice cultivars and experimental lines. Fitopatología Venezolana. 2008;21(2):51-8.
Rossman AY, Howard RJ, Valent B. Pyricularia grisea the correct name for the rice blast disease fungus. Mycologia. 1990;82(4):509-12.
Manjunatha B, Krishnappa M. Morphological characterization of Pyricularia oryzae causing blast disease in rice Oryza sativa L.) from different zones of Karnataka. Journal of Pharmacognosy and Phytochemistry. 2019;8(3):3749-53.
Ou SH. Rice Diseases: Commonwealth Mycological Institute. 2nd ed. Kew Surrey, England; 1985. 380 p.
Koutroubas SD, Katsantonis D, Ntanos DA, Lupotto E. Blast disease influence on agronomic and quality traits of rice varieties under Mediterranean conditions. Turkish Journal of Agriculture and forestry. 2009;33(5):487-94.
Kulmitra AK, Sahu N, Sahu MK, Kumar R, Kushram T, Sanath Kumar VB. Growth of Rice blast fungus Pyricularia oryzae (Cav.) on different solid and liquid media. International Journal of Current Microbiology and Applied Sciences. 2017;6(6):1154-60.
Cárdenas RM, Pérez N, Cristo E, González MC, Fabré L. Estudio sobre el comportamiento de líneas y variedades de arroz Oryza sativa Lin.) ante la infección por el hongo Pyricularia grisea Sacc. Cultivos Tropicales. 2005;26(4):83-7.
Cárdenas RM, Polón CR, Pérez N, Cristo E, Mesa S, Fabré L, et al. Relación entre la incidencia de la piriculariosis (Pyricularia grisea Sacc.) del arroz (Oryza sativa Lin.) y diferentes variables climáticas en el Complejo Agroindustrial Arrocero Los Palacios. Cultivos Tropicales. 2010;31(1):14-8.
Patiño L. La resistencia a fungicidas, una continúa amenaza al control de la Sigatoka Negra. Boletín Técnico Cenibanano. 2003;4:2-5.
Hua C, Li Y, Wang X, Kai K, Su M, Zhang D, et al. The effect of low and high molecular weight chitosan on the control of gray mold (Botrytis cinerea) on kiwifruit and host response. Scientia Horticulturae. 2019;246:700-9.
Sánchez-Domínguez D, Ríos MY, Castillo-Ocampo P, Zavala-Padilla G, Ramos-García M, Bautista-Baños S. Cytological and biochemical changes induced by chitosan in the pathosystem Alternaria alternata-tomato. Pesticide biochemistry and physiology. 2011;99(3):250-5.
Cortés-Higareda M, de Lorena Ramos-García M, Correa-Pacheco ZN, Del Río-García JC, Bautista-Baños S. Nanostructured chitosan/propolis formulations: characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon. 2019;5(5):e01776.
Sahariah P, Masson M. Antimicrobial chitosan and chitosan derivatives: a review of the structure-activity relationship. Biomacromolecules. 2017;18(11):3846-68.
Rodríguez Pedroso AT, Plascencia Jatomea M, Bautista Baños S, Cortez Rocha MO, Ramírez Arrebato MÁ. Actividad antifúngica in vitro de quitosanos sobre patógeno del arroz. Acta Agronomica. 2016;65(2):169-74.
Živković S, Stevanović M, Đurović S, Ristić D, Stošić S. Antifungal activity of chitosan against Alternaria alternata and Colletotrichum gloeosporioides. Pesticidi i fitomedicina. 2018;33(3-4):197-204.
Badawy ME, Rabea EI. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. International Journal of Carbohydrate Chemistry. 2011;2011.
Rabea EI, Badawy ME, Rogge TM, Stevens CV, Höfte M, Steurbaut W, et al. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Management Science. 2005;61(10):951-60.
Xu J, Zhao X, Han X, Du Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pesticide Biochemistry and Physiology. 2007;87(3):220-8.
Chen C-S, Liau W-Y, Tsai G-J. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. Journal of Food Protection. 1998;61(9):1124-8.
Jia Z, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydrate research. 2001;333(1):1-6.
Sashiwa H, Kawasaki N, Nakayama A, Muraki E, Yamamoto N, Zhu H, et al. Chemical modification of chitosan. 13. Synthesis of organosoluble, palladium adsorbable, and biodegradable chitosan derivatives toward the chemical plating on plastics. Biomacromolecules. 2002;3(5):1120-5.
Badawy ME, Rabea EI, Rogge TM, Stevens CV, Steurbaut W, Höfte M, et al. Fungicidal and insecticidal activity of O-acyl chitosan derivatives. Polymer bulletin. 2005;54(4):279-89.
Badawy M, Rabea E, Steurbaut W, Rogge T, Stevens C, Smagghe G, et al. Fungicidal activity of some O-acyl chitosan derivatives against grey mould Botrytis cinerea and rice leaf blast Pyricularia grisea. Communications in agricultural and applied biological sciences. 2005;70(3):215-8.
Ma G, Yang D, Tan H, Wu Q, Nie J. Preparation and characterization of N‐alkylated chitosan derivatives. Journal of applied polymer science. 2008;109(2):1093-8.
Másson M, Holappa J, Hjálmarsdóttir M, Rúnarsson ÖV, Nevalainen T, Järvinen T. Antimicrobial activity of piperazine derivatives of chitosan. Carbohydrate polymers. 2008;74(3):566-71.
Seyfarth F, Schliemann S, Elsner P, Hipler U-C. Antifungal effect of high-and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. International Journal of Pharmaceutics. 2008;353(1-2):139-48.
Zhong Z, Xing R, Liu S, Wang L, Cai S, Li P. Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydrate Research. 2008;343(3):566-70.
Tikhonov VE, Stepnova EA, Babak VG, Yamskov IA, Palma-Guerrero J, Jansson H-B, et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2 (3)-(dodec-2-enyl) succinoyl/-derivatives. Carbohydrate polymers. 2006;64(1):66-72.
Stössel P, Leuba JL. Effect of chitosan, chitin and some aminosugars on growth of various soilborne phytopathogenic fungi. Journal of Phytopathology. 1984;111(1):82-90.
Badawy ME, Rabea EI. Characterization and antimicrobial activity of water-soluble N-(4-carboxybutyroyl) chitosans against some plant pathogenic bacteria and fungi. Carbohydrate polymers. 2012;87(1):250-6.
Shimizu T, Jikumaru Y, Okada A, Okada K, Koga J, Umemura K, et al. Effects of a bile acid elicitor, cholic acid, on the biosynthesis of diterpenoid phytoalexins in suspension-cultured rice cells. Phytochemistry. 2008;69(4):973-81.
Agrawal GK, Rakwal R, Tamogami S, Yonekura M, Kubo A, Saji H. Chitosan activates defense/stress response (s) in the leaves of Oryza sativa seedlings. Plant Physiology and Biochemistry. 2002;40(12):1061-9.
Lin W, Hu X, Zhang W, Rogers WJ, Cai W. Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. Journal of Plant Physiology. 2005;162(8):937-44.
Nguyen TH, Thi TV, Nguyen T-T, Le TD, Vo DMH, Nguyen DH, et al. Investigation of chitosan nanoparticles loaded with protocatechuic acid (PCA) for the resistance of Pyricularia oryzae fungus against rice blast. Polymers. 2019;11(1):177.
Pham DC, Nguyen TH, Ngoc UTP, Le NTT, Tran TV, Nguyen DH. Preparation, characterization and antifungal properties of chitosan-silver nanoparticles synergize fungicide against Pyricularia oryzae. Journal of nanoscience and nanotechnology. 2018;18(8):5299-305.
Je J-Y, Kim S-K. Antimicrobial action of novel chitin derivative. Biochimica et Biophysica Acta (BBA)-General Subjects. 2006;1760(1):104-9.
El Hadrami A, Adam LR, El Hadrami I, Daayf F. Chitosan in plant protection. Marine drugs. 2010;8(4):968-87.
Zakrzewska A, Boorsma A, Brul S, Hellingwerf KJ, Klis FM. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryotic Cell. 2005;4(4):703-15.
Velásquez CL. Some potentialities of chitin and chitosan for uses related to agriculture in Latin America. Revista Científica UDO Agrícola. 2008;8(1):1-22.
Palma-Guerrero J, Huang I-C, Jansson H-B, Salinas J, Lopez-Llorca LV, Read ND. Chitosan permeabilizes the plasma membrane and kills cells of Neurosero J, Lopez‐Jimenez JA, Pérez‐Berná AJ, Huang I-C, Jansson H-B, Salinas J, et al. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Molecular microbiology. 2010;75(4):1021-32.
Cuero RG, Duffus E, Osuji G, Pettit R. Aflatoxin control in preharvest maize: effects of chitosan and two microbial agents. The Journal of Agricultural Science. 1991;117(2):165-9.
El Ghaouth A, Arul J, Asselin A, Benhamou N. Antifungal activity of chitosan on post-harvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycological research. 1992;96(9):769-79.
Sudarshan NR, Hoover DG, Knorr D. Antibacterial action of chitosan. Food Biotechnology. 1992;6(3):257-72.
Jo Y-K, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant disease. 2009;93(10):1037-43.