Characterization of indigenous arbuscular mycorrhizal fungi associated with coconut cultivation in Baracoa
Main Article Content
Abstract
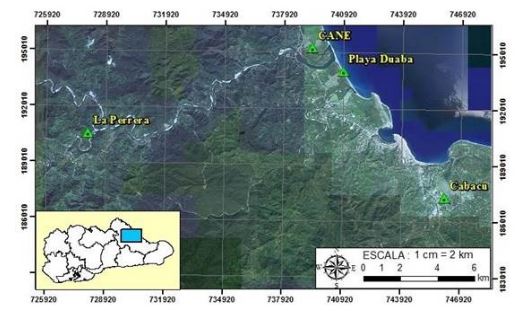
The 85 % of the national coconut production takes place in Baracoa, which is affected by the low availability of mineral fertilizers, which limits the obtaining of quality seedlings and the development of plantations. This geographical area is located in a Biosphere Reserve, Cuchillas del Toa, characterized by a high endemism, so it is a potential priority agricultural technology that allow its protection while increasing the productivity of the coconut tree. In order to have autochthonous strains with potential for use in the nutritional management of the coconut tree, the isolation and characterization of arbuscular mycorrhizal fungi (AMF) was carried out in four sites. A high abundance of AMF spores was found in Cabacú and Cane, while the lowest was in Playa Duana, with the highest percentage of species and morphotypes observed. A relative specificity of the Orders to which the characterized species and morphotypes belong in relation to the studied sites was observed. The results indicate that both the abundance and the species or morphotypes found depend on factors specific to each ecosystem, which determine the autochthonous community of AMF that is established. It is shown that despite the development of coconut cultivation in these sites, they constitute very well conserved ecosystems, mainly due to the few tasks and applications of fertilizers that are carried out, which preserves their ecological balance.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Those authors who have publications with this journal accept the following terms of the License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0):
You are free to:
- Share — copy and redistribute the material in any medium or format
- Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
The journal is not responsible for the opinions and concepts expressed in the works, they are the sole responsibility of the authors. The Editor, with the assistance of the Editorial Committee, reserves the right to suggest or request advisable or necessary modifications. They are accepted to publish original scientific papers, research results of interest that have not been published or sent to another journal for the same purpose.
The mention of trademarks of equipment, instruments or specific materials is for identification purposes, and there is no promotional commitment in relation to them, neither by the authors nor by the publisher.
References
Sudharmaidevi CR, Vinith V, Kavitha GV. Effect of potassium-sodium interaction on foliar nutrient concentration and nut quality of coconut (Cocos nucifera). Malaysian Journal of Soil Science. 2015;19:107-114. Available from: https://www.researchgate.net/publication/316963847
Sáenz L, Chan JL. Narvaez M, Oropeza C. Protocol for the micropropagation of coconut from plumule explants. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant Cell Culture Protocols. Methods in Molecular Biology, vol 1815. Humana Press, New York, NY; 2018. pp 161-170. doi: 10.1007/978-1-4939-8594-4_9.
Kaushik V, Chogale R, Mhaskar S. Single hair fiber assessment techniques to discriminate between mineral oil and coconut oil effect on hair physical properties. Journal of Cosmet Dermatol. 2021;20(4):1306-1317. doi:10.1111/jocd.13724.
Singh S, Lohani A, Mishra AK, Verma A. Formulation and evaluation of carrot seed oil-based cosmetic emulsions. J Cosmet Laser Ther. 2019;21(2):99-107. doi: 10.1080/14764172.2018.1469769.
Fang C, Paul CR, Day CH, Chang R, Kuo C, Ho T, Hsieh, DJ, Viswanadha VP, Kuo W, Huang C. Poria cocos (Fuling) targets TGFβ /Smad7 associated collagen accumulation and enhances Nrf2‐antioxidant mechanism to exert anti‐skin aging effects in human dermal fibroblasts. Environ Toxicol. 2020;36(5):729-736. doi:10.1002/tox.23075.
Li Y, Zheng Y,Zhang Y, Xu J, Gao G. Antioxidant activity of coconut (Cocos nucifera L.) protein fractions. Molecules 2018;23:707-718. doi: 10.3390/molecules23030707.
Petter PJ. Fatty acid sulphoalkyl amides and esters as cosmetic surfactants. Int J Cosmet Sci. 1984;6(5):249-60. doi: 10.1111/j.1467-2494.1984.tb00382.x.
Deen A, Visvanathan R, Wickramarachchi D, Marikkar N, Nammi S, Jayawardanae BC, Liyanagea R. Chemical composition and health benefits of coconut oil: an overview. J Sci Food Agric. 2021;101(6):2182-2193. doi 10.1002/jsfa.10870.
Neelakantan N, Seah JYH, van Dam RM. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Clinical Trials. Circulation. 2020;141(10):803-814. doi:10.1161/circulationaha.119.043052.
Teng M, Zhao YJ, Khoo AL, Yeo TC, Yong QW, Lim BP. Impact of coconut oil consumption on cardiovascular health: a systematic review and meta-analysis. Nutr Rev. 2020;78(3):249-259. doi:10.1093/nutrit/nuz074.
Wallace TC. Health Effects of Coconut Oil-A Narrative Review of Current Evidence. J Am Coll Nutr. 2019;38(2):97-107. doi:10.1080/07315724.2018.1497562
Bolivar-Telleria M, Turbay C, Favarato L, Carneiro T, de Biasi RS, Fernandes AAR, Santos AMC, Fernandes PMB. Second-Generation Bioethanol from Coconut Husk. BioMed Res Int. 2018;2018:4916497. doi:10.1155/2018/4916497.
Burns DT, Johnston EL, Walker MJ. Authenticity and the potability of coconut water - a critical review. J AOAC Int. 2020;103(3):800-806. doi: 10.1093/jaocint/qsz008.
Patil U, Benjaku, S. Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality. J Food Sci. 2018;83(8):2019-2027. doi:10.1111/1750-3841.14223.
Alvarado K, Blanco A, Martín J, Velásquez Y, Matos K. Situación socio-tecnológica-productiva del cultivo del cocotero en Baracoa, Cuba. Pastos y Forrajes. 2013;36 (2):252-261. Available from: https://payfo.ihatuey.cu/index.php?journal=pasto.
Ambili K, Thomas GV, Indu P, Gopal M, Gupta A. Distribution of arbuscular mycorrhizae associated with coconut and arecanut based cropping systems. Agricultural Research. 2012;1(4):338-345. Available from: https://www.researchgate.net/publication/249649900.
Rajeshkumar PP, Thomas GV, Gupta A, Gopal M. Diversity, richness and degree of colonization of arbuscular mycorrhizal fungi in coconut cultivated along with intercrops in high productive zone of Kerala, India. Symbiosis. 2015;65:125-141. doi 10.1007/s13199-015-0326-2.
Choi J, Summers W, Paszkowski U. Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu. Rev. Phytopathol. 2018;56:135-160. doi: 10.1146/annurev-phyto-080516-035521.
Müller L M, Harrison MJ. Phytohormones, miRNAs, and peptide signals integrate plant phosphorus status with arbuscular. mycorrhizal symbiosis. Curr Opin Plant Biol. 2019;50:132-139. doi: 10.1016/j.pbi.2019.05.004.
Luginbueh LH, Menard GN, Kurup S, Erp HV, Radhakrishnan GV, Breakspear A, Oldroyd GED, Eastmond PJ. The host plant synthesizes fatty acids in arbuscular mycorrhizal fungi. Science. 2017;356(6343):1175-1178. doi 10.1126/science.aan0081.
Abbaslou H, Bakhtiari S. Phytoremediation potential of heavy metals by two native pasture plants (Eucalyptus grandis and Ailanthus altissima) assisted with AMF and fibrous minerals in contaminated mining regions. Pollution. 2017;3(3):471-486. doi: 10.7508/pj.2017.03.0.
de la Noval B, Pérez E, Martínez B, León O, Martínez-Gallardo N, Délano-Frier J. Exogenous systemin has a contrasting effect on disease resistance in mycorrhizal tomato (Solanum lycopersicum) plants infected with necrotrophic or hemibiotrophic pathogens. Mycorrhiza. 2007;17(5):449-60. doi: 10.1007/s00572-007-0122-9.
Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019;10: 470. doi: 10.3389/fpls.2019.00470.
Lumini E, Pan J, Franco F, Cuihua Huang C, Bianciotto V, Xue X, Balestrini R, Tedeschi A. Native arbuscular mycorrhizal fungi characterization from saline lands in arid oases, northwest china. J. Fungi. 2020;6(2): 80-89. doi: 10.3390/jof6020080.
Entry IA, Rygiewicz PT, Watrud LS, Donnelly PK. Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Advances in Environmental Research. 2002;7:123-138. doi: 10.1016/S1093-0191(01)00109-5.
Cotton TEA. Arbuscular mycorrhizal fungal communities and global change: an uncertain future. FEMS Microbiol Ecol. 2018;94(11). doi:10.1093/femsec/fiy179.
Paneque PVM, Calaña NJM, Calderón VM, Borges BY, Hernández GTC, Caruncho CM. Manual de técnicas analíticas para análisis de suelo, foliar, abonos orgánicos y fertilizantes químicos [Internet]. 1st ed. La Habana, Cuba: Ediciones INCA; 2010 [cited 27/01/2016]. 157 p. Available from: http://mst.ama.cu/578/
Herrera Peraza RA, Furrazola E, Ferrer RL, Fernández Valle R, Torres Arias Y. Functional strategies of root hairs and arbuscular mycorrhizae in an evergreen tropical forest, Sierra del Rosario, Cuba. Revista CENIC Ciencias Biológicas. 2004;35(2):113-123. Available from: http://www.redalyc.org/articulo.oa?id=181226079010.
Schenck NC, Pérez Y. Manual for the Identification of VA Mycorrhizal Fungi. 3.rd Ed. Gainesville, FL: Synergistic Publ, 1990. Available from: https://www.worldcat.org/title/manual-for-the-identification-of-va-mycorrhizal-fungi/oclc/24677253.
Schüβler A, Walker C. Evolution of the 'Plant -Symbiotic' Fungal Phyllum,Glomeromycota. Evolution of fungi and fungal-like organisms. Chapter 7. The Mycota XIV. Pöggeler, S. y Wöstemeyer, J. (Eds.) © Springer‐Verlag Berlin Heidelberg, 2010, pp. 163-185. Available from: https://www.amf-phylogeny.com.
Blaszkowski J. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland [cited 20/09/2018]. Available from: https://www.agro.ar.szczecin.pl/~jblaszkowski.
Colección Internacional de Cultivos de Hongos Micorrizógenos Vesículo-Arbusculares (INVAM) [cited: 20/09/2018]. Available from: http://invam.caf.wvu.
Wang Q, Bao Y, Nan J, Xu D. AM fungal diversity and its impact across three types of mid-temperate steppe in Inner Mongolia, China. Mycorrhiza. 2020;30(1):97-108. doi: 10.1007/s00572-019-00926-x.
Pagano MC, Gupta VK. Overview of the Recent Advances in Mycorrhizal Fungi. In: Recent Advances on Mycorrhizal Fungi. Marcela C. Pagano (ed). Springer. 2016. doi 10.1007/978-3-319-24355-9.
Furrazola E, Heredia G, Olivera G, Sosa V. Efecto de comunidades nativas de hongos micorrizógenos arbusculares sobre el crecimiento de plántulas de maíz y sorgo. Acta Botánica Cubana. 2017;216(3):127-136. Available from: https://www.researchgate.net/publication/322234749.
Herrera RA, Bever J, Furrazola E, Ferrer RL, Herrera P. Estrategias funcionales de la diversidad fúngica micorrízica arbuscular: importancia del análisis del número de esporas de hongos glomeromicetos o de los biovolúmenes. Acta Botánica Cubana. 2019;218(2):143-159. Available from: https://www.researchgate.net/publication/339076979.
Torres Y, Ortega R, Nobre C, Furrazola E, Berbara RLL. Production of native arbuscular mycorrhizal fungi inoculum under different environmental conditions. Braz. J. Microbiol. 2017;48(1). doi: org/10.1016/j.bjm.2016.10.012.
Torres Y, Hernández, R, Furrazola E, Gutiérrez Y. Hongos micorrizógenos arbusculares (Glomeromycota) en el bosque de ciénaga El Embarcadero, en la provincia Mayabeque, Cuba. Acta Botánica Cubana. 2019;218(1):27-33. Available from: https://www.researchgate.net/publication/333457170.
Furrazola-Gómez E, Rodríguez- Rodríguez R, Torres-Arias Y, González- González S, Ortega-Fors R, Ley-Rivas JF. Hongos micorrizógenos arbusculares (Glomeromycotina) en ecosistemas naturales y agrícolas en la Reserva de la Biosfera Ciénaga de Zapata, Cuba. Acta Botánica Cubana. 2018;217(1):85-93. Available from: http://www.revistas.geotech.cu/index.php/abc
Furrazola E, Covacevich F, Torres AY, Rodríguez RRM, Ley RJF, Izquierdo K, Fernández VR, Louro BRL. Functionality of arbuscular mycorrhizal fungi in three plant communities in the Managed Floristic Reserve San Ubaldo-Sabanalamar, Cuba. International Journal Tropical Biology. 2015;63(2):341-356. Available from: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0034-77442015000200003.
Lara-Pérez LA, Oros-Ortega I, Córdova-Lara I, Estrada-Medina H, O’Connor-Sánchez A, Góngora-Castillo E, Sáenz-Carbonell L. Seasonal shifts of arbuscular mycorrhizal fungi in Cocos nucifera roots in Yucatán, México. Mycorrhiza. 2020;30(2-3):269-283. doi.org/10.1007/s00572-020-00944-0.
Peña-Venegas CP, Kuyper TW, Davison J, Jairus T, Vasar M, Stomph TJ, Struik PC, Öpik, M. Distinct arbuscular mycorrhizal fungal communities associate with different manioc landraces and Amazonian soils. Mycorrhiza. 2019;29(3):263-275. doi:10.1007/s00572-019-00891-5.
Seerangan K, Thangavelu M. Arbuscular Mycorrhizal and Dark Septate Endophyte Fungal Associations in South Indian Aquatic and Wetland Macrophytes. J Bot. 2014;2014:1-14. doi:10.1155/2014/173125.
Espinosa J, Ortea J, Moro L. Nueva especie de marginela del género Prunum Herrmannsen, 1852 (mollusca: neogastropoda: Marginellidae), del Parque Nacional Alejandro de Humboldt, sector Baracoa, Cuba. Academia Canaria de Ciencia. 2009;20 (4):19-22. Available from: https://www.researchgate.net/publication/294581730.
Lovera M, Cuenca G. Diversidad de hongos micorrízicos arbusculares (HMA) y potencial micorrízico del suelo de una sabana natural y una sabana perturbada de la Gran Sabana, Venezuela. Interciencia. 2007;32(2):108-114. Available from: http://www.redalyc.org/articulo.oa?id=33932206.
Pellegrino E, Gamper HA, Ciccolini V, Ercoli L. Forage Rotations Conserve Diversity of Arbuscular Mycorrhizal Fungi and Soil Fertility. Front Microbiol. 2020;10:2969. doi:10.3389/fmicb.2019.02969.
Teranishi T, Kobae Y. Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant. Plants. 2020;9(5):658. doi: 10.3390/plants9050658.