Management of phytopathogenic fungi on Oryza sativa with the application of Trichoderma asperellum
Main Article Content
Abstract
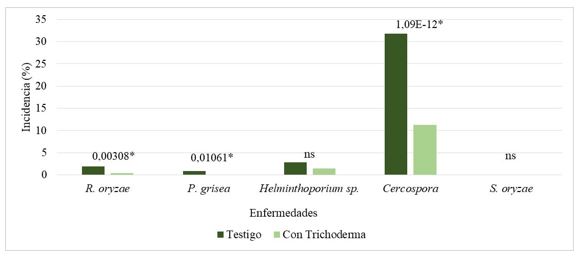
Fungal diseases in rice crop (Oryza sativa Lin.) are considered among the main causes of low cereal yields. In this sense, numerous studies indicate that the applications of Trichoderma asperellum Samuels, Lieckfeldt & Nirenberg strains to the soil in fields destined to the cultivation of rice, are promising for the control of numerous phytopathogenic fungi. The objective of the work was to evaluate the effect of the Ta 78 strain of T. asperellum against the most important diseases of rice cultivation under field conditions. For this, the strain of T. asperellum was sprayed in a terrace system during soil preparation, and a terrace was used as a production control. The severity and incidence of the main diseases caused by fungi were evaluated at various times and the number of plants, tillers and panicles per m2 was evaluated. R. solani, Helminthosporium sp. and Cercospora sp. were the fungi that most affected rice crop. The incidence and severity of the diseases evaluated in the treatment with T. asperellum, decreased significantly with respect to the control treatment, regardless of the time of the evaluation. On the other hand, the number of tillers and spikes was significantly higher in the plot treated with biological control.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Those authors who have publications with this journal accept the following terms of the License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0):
You are free to:
- Share — copy and redistribute the material in any medium or format
- Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
The journal is not responsible for the opinions and concepts expressed in the works, they are the sole responsibility of the authors. The Editor, with the assistance of the Editorial Committee, reserves the right to suggest or request advisable or necessary modifications. They are accepted to publish original scientific papers, research results of interest that have not been published or sent to another journal for the same purpose.
The mention of trademarks of equipment, instruments or specific materials is for identification purposes, and there is no promotional commitment in relation to them, neither by the authors nor by the publisher.
References
Martínez S, Bao L, Escalante F. Manual de identificación de enfermedades y plagas en el cultivo de arroz. Instituto Nacional de Investigación Agropecuaria. 2018;(116).
Pérez_Iglesias HI, García-Batista RM. Principales enfermedades que afectan al cultivo del arroz en Ecuador y alternativas para su control. Revista Científica Agroecosistemas. 2018;6(1):16-27.
Bhumi-Narsimha R, Kamma-Venkata S, Amballa H. In vitro screening for antagonistic potential of seven species of Trichoderma against different plant pathogenic fungi. Research Journal of Biology. 2014;2:29-36.
Companioni González B, Domínguez Arizmendi G, García Velasco R, Companioni González B, Domínguez Arizmendi G, García Velasco R. Trichoderma: su potencial en el desarrollo sostenible de la agricultura. Biotecnología Vegetal. 2019;19(4):237-48.
Coca BM, Infante D, Reyes Y, González I, Peteira B, Arias Y, et al. Bases científico - metodológicas para la selección, caracterización y uso de aislamientos de trichoderma como agente de control biológico del tizón de la vaina (rhizoctonia solani kühn) en arroz. Anales de la Academia de Ciencias de Cuba [Internet]. 2017;7(1). [cited 14/09/2022] Available from: http://revistaccuba.sld.cu/ index.php/revacc/article/view/470
Singh U, Singh S, Malviya D, Chaurasia R, Imran M, Rai J. Harnessing biocontrol potential of Trichoderma harzianum for control of Meloidogyne incognita in tomato. Indian Phytopathol. 2017;70:331-5.
De Palma M, Salzano M, Villano C, Aversano R, Lorito M, Ruocco M, et al. Transcriptome reprogramming, epigenetic modifications and alternative splicing orchestrate the tomato root response to the beneficial fungus Trichoderma harzianum. Horticulture Research [Internet]. 2019;6(5). [cited 14/09/2022] doi:10.1038/s41438-018-0079-1.
Infante D, Martínez B, Peteira B, Reyes Y, Herrera A. Identificación molecular y evaluación patogénica de trece aislamientos de Trichoderma spp. frente a Rhizoctonia solani Kühn. Biotecnología Aplicada. 2013;30(1):17-22.
Hernández A, Pérez J, Bosch D, Castro N. Clasificación de los suelos de Cuba 2015. Mayabeque, Cuba: Ediciones INCA; 2015.
MINAG. Instructivo Técnico para el cultivo del arroz. LaHabana: Instituto de Investigaciones del Arroz; 2015 p.115.
Estándar. Evaluation System for Rice (SES) [Internet]. International Rice Research Institute (IRRI); 2016. Available from: http://www.knowledgebank.irri.org/images/ docs/rice-standard-evaluation-system.pdf
Townsend GR. Methods for estimating losses caused bydiseases in fungicide experiments. Plant Disease Reporter. 1943;27:340-3.
Folguera-Montiel M, Rodríguez-Morales S, Herrera-Isla L,Sánchez-Rodríguez R. Influencia de diferentes métodos de plantación en la incidencia de las pudriciones radicales de la yuca (“Manihot esculenta Crantz”). Cuadernos de fitopatología: Revista técnica de fitopatología y entomología. 2011;28(108):23-7.
Cruz-Triana A, Rivero-González D, Martínez-Coca B,Echevarría-Hernández A, Tania-Rodríguez A. Evaluación de la actividad antifúngica de Trichoderma asperellum Samuels ante patógenos fúngicos que afectan al cultivo de la soya (Glycine max L.). Cultivos Tropicales. 2017;38(4):15-21.
Cruz-Triana A, Rivero-González D, Infante-Martínez D,Echevarría-Hernández A, Martínez-Coca B, Cruz-Triana A, et al. Manejo de hongos fitopatógenos en Phaseolus vulgaris L. con la aplicación de Trichoderma asperellum Samuels, Lieckfeldt & Nirenberg. Revista de Protección Vegetal [Internet]. 2018;33(3). [cited 14/09/2022] Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&p id=S1010-27522018000300004&lng=es&nrm=iso&tlng=en
Dotson BR, Soltan D, Schmidt J, Areskoug M, Rabe K, Swart C, et al. The antibiotic peptaibol alamethicin from Trichoderma permeabilises Arabidopsis root apical meristem and epidermis but is antagonised by cellulaseinduced resistance to alamethicin. BMC Plant Biology. 2018;18(1):165. doi:10.1186/s12870-018-1370-x16
Pineda-Insuasti JA, Benavides-Sotelo EN, Duarte-TrujilloAS, Burgos-Rada CA, Soto-Arroyave CP, Pineda-Soto CA, et al. Producción de biopreparados de Trichoderma spp: una revisión. ICIDCA. Sobre los Derivados de la Caña de Azúcar. 2017;51(1):47-52.
Malmierca MG, Barua J, McCormick SP, Izquierdo-BuenoI, Cardoza RE, Alexander NJ, et al. Novel aspinolide production by Trichoderma arundinaceum with a potential role in Botrytis cinerea antagonistic activity and plant defence priming. Environmental Microbiology.
;17(4):1103-18. doi:https://doi.org/10.1111/1462-2920. 12514
Companioni-González B, Domínguez-Arizmendi G, García-Velasco R, Companioni-González B, DomínguezArizmendi G, García-Velasco R. Trichoderma: su potencial en el desarrollo sostenible de la agricultura. Biotecnología Vegetal. 2019;19(4):237-48.
Benhamou N. Elicitor-Induced Resistance in TomatoPlants Against Fungal Pathogens: Ultrastructure and Cytochemistry of the Induced Response. Scanning Microscopy [Internet]. 1995;9(3). Available from: https:// digitalcommons.usu.edu/microscopy/vol9/iss3/22
Cortés C, Gutiérrez A, Olmedo V, Inbar J, Chet I, HerreraEstrella A. The expression of genes involved in parasitism by Trichoderma harzianum is triggered by a diffusible factor. Molecular and General Genetics MGG. 1998;260(2):218-25. doi:10.1007/s004380050889
Schirawski J, Perlin MH. Plant-Microbe Interaction 2017The Good, the Bad and the Diverse. International Journal of Molecular Sciences. 2018;19(5):1374. doi:10.3390/ ijms19051374