Beneficial microorganisms antagonistic to Curvularia petersonii, which causes foliar lesions in sugar cane
Main Article Content
Abstract
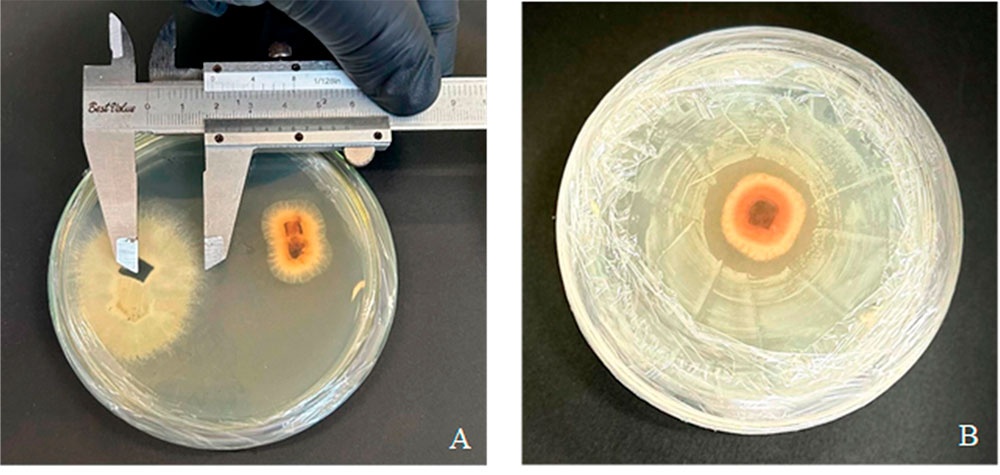
Foliar necrosis in sugarcane caused by phytopathogenic fungi is a serious problem; as a biocontrol alternative, beneficial microorganisms could offer a sustainable solution. The objective of this study was to evaluate the antagonistic effect of beneficial microorganisms against Curvularia petersonii, a phytopathogenic fungus responsible for foliar necrosis in sugar cane cultivation. A sequenced strain with 99.60% identity for C. petersonii (Accession No. NR_158448.1) to evaluate the percentage of inhibition (Trichoderma harzianum, Trichoderma asperellum, Bacillus subtilis, and Pseudomonas fluorescens) with 5 replicates and their respective controls over 16 days of evaluation. The mathematical modeling technique of exponential growth was used to model the development of C. petersonii under in vitro conditions and in the presence of antagonistic microorganisms using the Lotka-Volterra for dual crop confrontation. The results support that the beneficial microorganisms used had an antagonistic inhibitory effect on the phytopathogenic fungus C. petersonii, concluding that these microorganisms have advantages as biocontrol agents against C. petersonii.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Those authors who have publications with this journal accept the following terms of the License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0):
You are free to:
- Share — copy and redistribute the material in any medium or format
- Adapt — remix, transform, and build upon the material
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- NonCommercial — You may not use the material for commercial purposes.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
The journal is not responsible for the opinions and concepts expressed in the works, they are the sole responsibility of the authors. The Editor, with the assistance of the Editorial Committee, reserves the right to suggest or request advisable or necessary modifications. They are accepted to publish original scientific papers, research results of interest that have not been published or sent to another journal for the same purpose.
The mention of trademarks of equipment, instruments or specific materials is for identification purposes, and there is no promotional commitment in relation to them, neither by the authors nor by the publisher.
References
Vera J, Lazo R, Barzallo D, Gavin C. Caracterización química y degradabilidad in situ de residuos orgánicos como alternativa alimenticia para bovinos. Ecuadorian Sci J. 2021;5(4):1-14. Available from: https://doi.org/10.21930/rcta.vol25_num1_art:3306274
Kong C-Y, Wickramasinghe KP, Xu C-H, Mao J, Liu H-B, Kumar T, et al. Recent advances in sugarcane leaf scald disease: Pathogenic insights and sustainable management approaches. Plants [Internet]. 2025;14(4):508. Available from: https://doi.org/10.3390/plants14040508
Jernisha J, Poorniammal R, Sivakumar U, Harish S, Sethuraman K. Plant growth promoting microorganisms and emerging biotechnological approaches for sugarcane disease management. J Pure Appl Microbiol [Internet]. 2024;18(4). Available from: https://doi.org/10.22207/JPAM.18.4.27
Ahmad S, Wang M, Zhang H, Deng Y, Liang Q, He B, et al. Synergistic application of biochar and lime modulates rhizosphere microbiome, suppresses pathogens, and enhances disease resistance in sugarcane. BMC Microbiology [Internet]. 2025;25(1):622. Available from: https://doi.org/10.1186/s12866-025-04355-z
Guo Z-J, Amenyogbe MK, Chen S-Q, Rashad YM, Deng J-X, Luo H. Morphological and phylogenetic analyses reveal two novel species of Curvularia (Pleosporales, Pleosporaceae) from southern China. MycoKeys [Internet]. 2025;120:139. Available from: https://doi.org/10.3897/mycokeys.120.156570
Srivastava AK, Khan M, Li X, Misra P, Ashish, Kumar A, et al. Exploring the impact of Curvularia pathogens on medicinal and aromatic plants: insights into history, pathogenicity, and host-pathogen interactions. World J Microbiol Biotechnol [Internet]. 2025;41(7):252. Available from: https://doi.org/10.1007/s11274-025-04421-8
Singh V, Lakshman DK, Roberts DP, Ismaiel A, Abhishek A, Kumar S, et al. Fungal species causing maize leaf blight in different agro-ecologies in India. Pathogens [Internet]. 2021;10(12):1621. Available from: https://doi.org/10.3390/pathogens10121621
Yusifova A, Asadova B, Aslanova S. Evaluation of phytopathogenic fungi according to the degree of danger. Adv Stud Biol [Internet]. 2025;17(1):27-36. Available from: https://doi.org/10.12988/asb.2025.91987
Tongsri V, Apithanasakulngeon P, Songkumarn P, Suttiviriya P, Chanchula N. Fungicide resistance of chrysanthemum fungal pathogens and control of leaf spot disease in pot conditions using effective fungicides. Int J Agric Technol [Internet]. 2025;21(4):1577-96. Available from: https://doi.org/10.63369/ijat.2025.21.4.1577-1596
Rodríguez JHV, Sarango Y, Aveiga M del RV, Mata JDO, Sevilla-Carrasco JD, Cuesta JMD, et al. Effect of herbicides on the growth of beneficial microorganisms in rhizospheric soil. Rev La Fac Agron La Univ Del Zulia [Internet]. 2025;42(2). Available from: https://doi.org/10.47280/RevFacAgron(LUZ).v42.n2.VI
Karamchandani BM, Chakraborty S, Dalvi SG, Satpute SK. Chitosan and its derivatives: Promising biomaterial in averting fungal diseases of sugarcane and other crops. J Basic Microbiol [Internet]. 2022;62(5):533-54. Available from: https://doi.org/10.1002/jobm.202100613
Rodríguez JHV, Mercedes MMM, Fernanda AFA, Enrique BEB, Liliana RLR. Microorganismos benéficos, bioestimuladores de la germinación y emergencia de semillas de maíz (Zea mays L.). Multidiscip Collab J [Internet]. 2025;3(3):58-69. Available from: https://doi.org/10.70881/mcj/v3/n3/70
Carpio M, Vera J, Yugsan F, Gavin C, Barzallo D. Biofertilizer enriched with Paenibacillus polymyxa and Trichoderma sp. for radish cultivation. Revista Caatinga [Internet]. 2025;38:e13759-e13759. Available from: https://doi.org/10.1590/1983-21252025v3813759rc
Vera Rodríguez JH, Barzallo D, Villamar Aveiga M del R, Barcia-Anchundia JX. Biostimulant effect of microorganisms on in vitro germination of hybrid pepper seeds. Rev Cienc y Tecnol Agropecu. 2024;25(1).
Ren Q, Khan A, Zhang J, Bao Y, Khan MT, Wang J, et al. Fungal community dynamics associated with the outbreaks of sugarcane root rot disease. Microbiol Spectr [Internet]. 2024;12(2):e03090-23. Available from: https://doi.org/10.1128/spectrum.03090-23
Jeres-Caguana GA, Montaño-Roldan VL, Ordoñez-Zuñiga NL, Vera-Rodriguez JH, Lucas-Vidal LR. Efecto biorremediador de la espirulina y Trichoderma spp. en suelo contaminado con plomo (Pb). Multidiscip Collab Journal [Internet]. 2025;3(2):1-12. Available from: https://doi.org/10.70881/mcj/v3/n2/48
Cortés-Hernández F del C, Alvarado-Castillo G, Sánchez-Viveros G. Trichoderma spp., una alternativa para la agricultura sostenible: una revisión. Revista Colomb Biotecnol [Internet]. 2023;25(2):73-87. Available from: https://doi.org/10.15446/rev.colomb.biote.v25n2.111384
Peralta JT, Guerra EG, Pruna DC, Erazo VS. Evaluación in vitro del antagonismo de cepas de Trichoderma contra hongos fitopatógenos foliares del cultivo de banano (Musa spp.). Agroindustrial Sci [Internet]. 2025;15(2):143-53. Available from: http://doi.org/10.17268/agroind.sci.2025.02.06
Martínez-Canto OJ, Cristóbal-Alejo J, Tun-Suárez JM, Reyes-Ramírez A. Detección de genes Epl1 y Sm1 en Trichoderma spp. antagonistas contra hongos fitopatógenos. Ecosistemas y Recursos Agropecurios [Internet]. 2021;8(2). Available from: https://doi.org/10.19136/era.a8n2.2791
Álvarez-García J-A, Santoyo G, del Carmen Rocha-Granados M. Pseudomonas fluorescens: Mecanismos y aplicaciones en la agricultura sustentable. Revista Latinoamericana Recursos Naturales [Internet]. 2020;16(1):1-10. Available from: https://revista.itson.edu.mx/index.php/rlrn/article/view/286
de los M Orberá T, de Jesús Serrat M, Ortega E. Potential applications of Bacillus subtilis strain SR/B-16 for the control of phytopathogenic fungi in economically relevant crops. Biotecnol Apl [Internet]. 2014;31(1):7-12. Available from: https://www.medigraphic.com/cgi-bin/new/resumenI.cgi?IDARTICULO=48662