Efecto del pH en la bioprotección ejercida por algunas cepas de hongos micorrízicos arbusculares
Contenido principal del artículo
Resumen
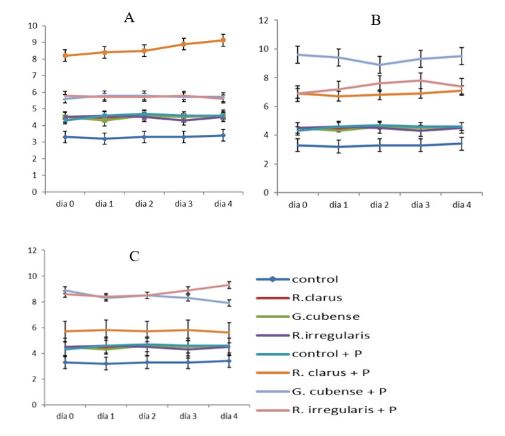
Las micorrizas arbusculares han sido ampliamente descritas como favorecedoras del crecimiento vegetal y realizan cambios físicos, bioquímicos y fisiológicos en las raíces que conducen a un mejor estado general de la planta y contribuyen a aliviar las situaciones de estrés de carácter abiótico y biótico. Producto de sus múltiples beneficios se ha ido incrementando paulatinamente su uso en la agricultura cubana. El sistema de recomendación de cepas de carácter generalistas del INCA se basa, fundamentalmente, en el tipo de suelo y su fertilidad asociada. Conocer cómo se integra el efecto de bioprotección de diferentes cepas con el pH puede contribuir a dilucidar si el efecto se asocia a una cepa “per se” o depende de la efectividad de las mismas. Para ello se diseñó un experimento utilizando un suelo Argissolo rojo-amarillento, ajustando las concentraciones de Ca2+ y Mg2+ a un único nivel y tres valores de pH (5,5; 6,5 y 7,2) con el objetivo de conocer si las diferencias en el pH también influyen en la bioprotección ejercida por estas cepas. Se utilizaron las cepas Rhizophagus irregularis, Glomus cubense y Rizophagus clarus recomendadas para diferentes rangos de pH y se usó como patógeno Fusarium oxysporum f. sp. phaseoli que fue inoculado en plantas de frijol de 21 días de edad. Las cepas originaron respuestas diferenciadas dependientes del pH en la intensidad de la colonización, la bioprotección ejercida y la inducción activa de peroxidasas, indicando que el efecto de bioproteccion se asoció a la efectividad que presentaba cada cepa en uno u otro pH.
Detalles del artículo

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes de la Licencia CC Reconocimiento-NoComercial 4.0 Internacional (CC BY-NC 4.0):
Usted es libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia.
Bajo las condiciones siguientes:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
La revista no se responsabiliza con las opiniones y conceptos emitidos en los trabajos, son de exclusiva responsabilidad de los autores. El Editor, con la asistencia del Comité de Editorial, se reserva el derecho de sugerir o solicitar modificaciones aconsejables o necesarias. Son aceptados para publicar trabajos científico originales, resultados de investigaciones de interés que no hayan sido publicados ni enviados a otra revista para ese mismo fin.
La mención de marcas comerciales de equipos, instrumentos o materiales específicos obedece a propósitos de identificación, no existiendo ningún compromiso promocional con relación a los mismos, ni por los autores ni por el editor.
Citas
Rivero J, Gamir J, Aroca R, Pozo MJ, Flors V. Metabolic transition in mycorrhizal tomato roots. Frontiers in Microbiology [Internet]. 2015 [cited 05/07/2022]; doi:10.3389/fmicb.2015.00598
Lanfranco L, Bonfante P, Genre A. The Mutualistic Interaction between Plants and Arbuscular Mycorrhizal Fungi. Microbiology Spectrum. 2016;1-20. doi:10.1128/microbiolspec.FUNK-0012-2016
Pozo MJ, López-Ráez JA, Azcón-Aguilar C, García-Garrido JM. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytologist. 2015;205(4):1431-6. doi:https://doi.org/10.1111/nph.13252
Helgason T, Fitter AH. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). Journal of Experimental Botany. 2009;60(9):2465-80. doi:10.1093/jxb/erp144
Smith FA, Smith SE. How harmonious are arbuscular mycorrhizal symbioses? Inconsistent concepts reflect different mindsets as well as results. The New Phytologist. 2015;205(4):1381-4.
Espinosa R, Felix F, Martinez L, Cañizares P, Yakelín R, Ortega E. Manejo, integración y beneficios del biofertilizante micorrízico EcoMic (r) en la producción agrícola [Internet]. 2020. INCA. Mayabeque, Cuba; 2020. 155 p.
Hashem A, Abd_Allah EF, Alqarawi AA, Egamberdieva D. Arbuscular Mycorrhizal Fungi and Plant Stress Tolerance. In: Egamberdieva D, Ahmad P, editors. Plant Microbiome: Stress Response [Internet]. Singapore: Springer; 2018 [cited 11/07/2022]. p. 81-103. doi:10.1007/978-981-10-5514-0_4
Lanfranco L, Fiorilli V, Gutjahr C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytologist. 2018;220(4):1031-46. doi:https://doi.org/10.1111/nph.15230
Venturi V, Keel C. Signaling in the Rhizosphere. Trends in Plant Science. 2016;21(3):187-98. doi:10.1016/j.tplants.2016.01.005
Herrera-Peraza RA, Hamel C, Fernández F, Ferrer RL, Furrazola E. Soil-strain compatibility: the key to effective use of arbuscular mycorrhizal inoculants? | SpringerLink. 2011;21:183-193.
Wendland-Ferreira A. Comunicación personal EMBRAPA [Internet]. 2020 [19/07/2022]. Available from: https://www.embrapa.br/agencia-de-informacao-tecnologica/inicial
Fernández F, Dell’Amico J, Pérez Y. Inoculante micorrizógeno líquido. Oficina Cubana de la Propiedad Industrial. 2009;23479.
Pozo MJ, Cordier C, Dumas‐Gaudot E, Gianinazzi S, Barea JM, Azcón‐Aguilar C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. Journal of Experimental Botany. 2002;53(368):525-34. doi:10.1093/jexbot/53.368.525
Vierheilig H, Schweiger P, Brundrett M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots†. Physiologia Plantarum. 2005;125(4):393-404. doi:https://doi.org/10.1111/j.1399-3054.2005.00564.x
Giovannetti M, Mosse B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. The New Phytologist. 1980;84(3):489-500.
Trouvelot A, Kough JL, Gianinazzi-Pearson V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthode d’estimation ayant une signification fonctionnelle. In 1986 [cited 11/07/2022]. p. 217-21. Available from: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=8758731
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1):248-54. doi:10.1016/0003-2697(76)90527-3
Heitefuß R, Williams PH. Oxidative enzymes. In: HEITEFUSS, R., and P. H. WILLIAMS. In: Physiological Plant Pathology [Internet]. New York: Springer Science & Business Media; 2012. p. 617-27. Available from: https://books.google.es/books?hl=es&lr=&id=hfLuCAAAQBAJ&oi=fnd&pg=PA1&dq=Oxidative+enzymes.+In:+HEITEFUSS,+R.,+and+P.+H.+WILLIAMS+(eds.).+Physiological+Plant+Pathology.+Encyclopedia+of+Plant+Pathology&ots=fu0GAetnMC&sig=pBM1iYFG95tlKKzYoQXrcb4q7UQ#v=onepage&q&f=false
Sun-Xue G, Tang M. Comparison of four routinely used methods for assessing root colonization by arbuscular mycorrhizal fungi. Botany. 2012;90:1073-83. doi:10.1139/b2012-084
Rivera R, González PJ, Hernández A, Martín G, Ruiz L, Fernández K, et al. La importancia del ambiente edáfico y del pH sobre la efectividad y la recomendación de cepas eficientes de HMA para la inoculación de los cultivos. In: VIII Congreso de la Sociedad Cubana de la Ciencia del Suelo. 2015.
Kanwal S, Bano A, Malik RN. Role of arbuscular mycorrhizal fungi in phytoremediation of heavy metals and effects on growth and biochemical activities of wheat (Triticum aestivum L.) plants in Zn contaminated soils. African Journal of Biotechnology. 2016;15(20):872-83. doi:10.4314/ajb.v15i20
Chaudhary VB, O’Dell TE, Rillig MC, Johnson NC. Multiscale patterns of arbuscular mycorrhizal fungal abundance and diversity in semiarid shrublands. Fungal Ecology. 2014;12:32-43. doi:10.1016/j.funeco.2014.06.003
Alguacil M del M, Torres MP, Montesinos-Navarro A, Roldán A. Soil Characteristics Driving Arbuscular Mycorrhizal Fungal Communities in Semiarid Mediterranean Soils. Applied and Environmental Microbiology. 2016;82(11):3348-56. doi:10.1128/AEM.03982-15
Wild A. Condiciones del suelo y desarrollo de las plantas según Russell. In: La población microbiana del suelo. Mur di-Prensa. Madrid, España: Ed. Mundi-Prensa; 1992. p. 471-94.
Igiehon NO, Babalola OO. Below-ground-above-ground Plant-microbial Interactions: Focusing on Soybean, Rhizobacteria and Mycorrhizal Fungi. The Open Microbiology Journal. 2018;12:261-79. doi:10.2174/1874285801812010261
Jamiołkowska A, Księżniak A, Gałązka A, Hetman B, Kopacki M, Skwaryło-Bednarz B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: a review. International Agrophysics. 2018;32(1):133-40. doi:10.1515/intag-2016-0090
Bücking H, Kafle A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy. 2015;5(4):587-612. doi:10.3390/agronomy5040587
Choudhary KK, Chaudhary N, Agrawal S, Agrawal M. Reactive Oxygen Species in Plants: Boon Or Bane - Revisiting the Role of ROS. In: Reactive oxygen species: generation, damage and quenching in plants during stress. In: Singh V.P. Wiley, Hoboken. NJ, USA: John Wiley & Sons; 2017.
Ouzounidou G, Skiada V, Papadopoulou KK, Stamatis N, Kavvadias V, Eleftheriadis E, et al. Effects of soil pH and arbuscular mycorrhiza (AM) inoculation on growth and chemical composition of chia (Salvia hispanica L.) leaves. Brazilian Journal of Botany. 2015;38(3):487-95. doi:10.1007/s40415-015-0166-6
Öpik M, Zobel M, Cantero JJ, Davison J, Facelli JM, Hiiesalu I, et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza. 2013;23(5):411-30. doi:10.1007/s00572-013-0482-2
Gilbert L, Johnson D. Chapter Four - Plant-Plant Communication Through Common Mycorrhizal Networks. In: Becard G, editor. Advances in Botanical Research [Internet]. Academic Press; 2017 [cited 19/07/2022]. p. 83-97. (How Plants Communicate with their Biotic Environment; vol. 82). doi:10.1016/bs.abr.2016.09.001
Lambers H, Albornoz F, Kotula L, Laliberté E, Ranathunge K, Teste FP, et al. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant and Soil. 2018;424(1):11-33. doi:10.1007/s11104-017-3427-2